Exam 13: The Solid State
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
A metal crystallizes in a face-centered cubic lattice. The radius of the atom is 214 pm and the density of the element is 2.63 g/cm3. What is the identity of the metal?
(Multiple Choice)
4.8/5  (43)
(43)
The bandgap of ZnTe is 218 kJ/mol. What is the maximum wavelength of light that can excite an electron transition across the band gap? (h = 6.626 × 10-34 J·s; c = 3.000 × 108 m/s)
(Multiple Choice)
4.7/5  (45)
(45)
Which equation represents the number of atoms in a body-centered cubic unit cell?
(Multiple Choice)
4.8/5  (34)
(34)
A sketch of a phase diagram is given below. 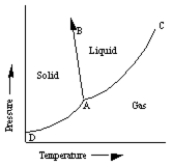 Which statement about this diagram is not true?
Which statement about this diagram is not true?
(Multiple Choice)
4.8/5  (30)
(30)
Silver chloride adopts the sodium chloride (rock salt) structure. The length of a unit cell edge is 555 pm. What is the density of AgCl?
(Multiple Choice)
4.8/5  (35)
(35)
What is the distance, in atomic radii, along any edge of a body-centered cubic unit cell?
(Multiple Choice)
4.9/5  (38)
(38)
In what type of unit cell are the "A" atoms arranged in the given unit cell? 2 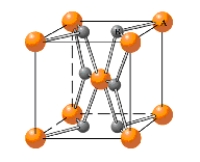
(Multiple Choice)
4.9/5  (28)
(28)
Showing 61 - 67 of 67
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)