Exam 10: An Introduction to Organic Chemistry
Exam 1: Chemistry - Methods and Measurement104 Questions
Exam 2: The Structure of the Atom and the Periodic Table115 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds93 Questions
Exam 4: Calculations, chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter: Gases, liquids, and Solids76 Questions
Exam 6: Solutions69 Questions
Exam 7: Energy,rate and Equilibrium67 Questions
Exam 8: Acids and Bases67 Questions
Exam 9: The Nucleus, radioactivity, and Nuclear Medicine86 Questions
Exam 10: An Introduction to Organic Chemistry104 Questions
Exam 11: The Unsaturated Hydrocarbons: Alkenes, alkynes, and Aromatics74 Questions
Exam 12: Alcohols, phenols, thiols, and Ethers69 Questions
Exam 13: Aldehydes and Ketones63 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives83 Questions
Exam 15: Amines and Amides82 Questions
Exam 16: Carbohydrates81 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems78 Questions
Exam 18: Protein Structure and Function75 Questions
Exam 19: Enzymes74 Questions
Exam 20: Introduction to Molecular Genetics85 Questions
Exam 21: Carbohydrate Metabolism76 Questions
Exam 22: Aerobic Respiration and Energy Production75 Questions
Exam 23: Fatty Acid Metabolism75 Questions
Select questions type
Functional groups are primarily responsible for the chemical properties of molecules in which they are found.Which of the following compounds have the same functional group,and would therefore have the same chemical properties? 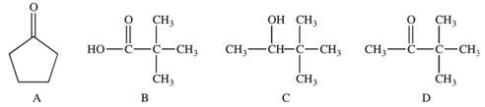
(Multiple Choice)
4.7/5  (29)
(29)
What is the name of the alkyl group represented by the structure below? 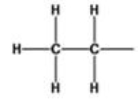
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following molecular formulas is not that of an alkane or cycloalkane?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is a possible product of the halogenation reaction shown below? 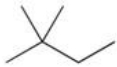
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following compounds would be named 3-ethyl-2-methylhexane?
(Multiple Choice)
4.8/5  (32)
(32)
Organic compounds tend to be more soluble in hexane than inorganic compounds.
(True/False)
4.8/5  (39)
(39)
The large number of organic compounds is due in part to the ability of these compounds to form constitutional isomers.Which of the following pairs of compounds are related as constitutional isomers?
(Multiple Choice)
4.7/5  (43)
(43)
The functional group shown below is found in what type of compound? 
(Multiple Choice)
4.9/5  (32)
(32)
Which bonding pattern is NOT typical of carbon atoms in organic compounds?
(Multiple Choice)
4.9/5  (41)
(41)
Eicosane is the name of the organic compound with the condensed formula CH3(CH2)18CH3.What does the prefix "eicos" specifically indicate about the structure of this compound?
(Multiple Choice)
4.8/5  (44)
(44)
The reaction shown below can be used to prepare alkyl halides.Which of the following best describes the type of reaction illustrated? 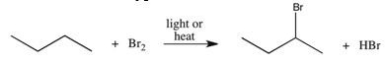
(Multiple Choice)
4.8/5  (31)
(31)
Which equation properly represents the complete combustion of propane,C3H8?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is NOT a typical property of inorganic compounds?
(Multiple Choice)
4.7/5  (44)
(44)
The chair conformation of cyclohexane is more stable than the boat conformation.
(True/False)
4.8/5  (38)
(38)
How do the different conformations of an alkane molecule arise?
(Multiple Choice)
5.0/5  (37)
(37)
Showing 61 - 80 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)