Exam 3: Structure and Properties of Ionic and Covalent Compounds
Exam 1: Chemistry - Methods and Measurement104 Questions
Exam 2: The Structure of the Atom and the Periodic Table115 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds93 Questions
Exam 4: Calculations, chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter: Gases, liquids, and Solids76 Questions
Exam 6: Solutions69 Questions
Exam 7: Energy,rate and Equilibrium67 Questions
Exam 8: Acids and Bases67 Questions
Exam 9: The Nucleus, radioactivity, and Nuclear Medicine86 Questions
Exam 10: An Introduction to Organic Chemistry104 Questions
Exam 11: The Unsaturated Hydrocarbons: Alkenes, alkynes, and Aromatics74 Questions
Exam 12: Alcohols, phenols, thiols, and Ethers69 Questions
Exam 13: Aldehydes and Ketones63 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives83 Questions
Exam 15: Amines and Amides82 Questions
Exam 16: Carbohydrates81 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems78 Questions
Exam 18: Protein Structure and Function75 Questions
Exam 19: Enzymes74 Questions
Exam 20: Introduction to Molecular Genetics85 Questions
Exam 21: Carbohydrate Metabolism76 Questions
Exam 22: Aerobic Respiration and Energy Production75 Questions
Exam 23: Fatty Acid Metabolism75 Questions
Select questions type
Which statement is TRUE concerning the solubility of a substance in H2O?
(Multiple Choice)
4.8/5  (41)
(41)
Which one of the following is NOT true about elements that form cations?
(Multiple Choice)
4.8/5  (39)
(39)
According to the VSEPR theory,what is the geometry (shape)around the phosphorus atom in the Lewis structure shown below? 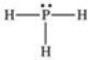
(Multiple Choice)
4.9/5  (33)
(33)
What kind of bond results when electron transfer occurs between atoms of two different elements?
(Multiple Choice)
4.8/5  (36)
(36)
Predict the formula of the compound formed when ions of sodium and sulfur combine.
(Multiple Choice)
5.0/5  (38)
(38)
What kind of bonding exists in substances that consist of discrete molecules?
(Multiple Choice)
4.9/5  (41)
(41)
In what region on the periodic table are elements with the lowest electronegativity values found?
(Multiple Choice)
4.8/5  (36)
(36)
A certain compound has a very high melting point,and when it dissolves in water the solution conducts electricity.Which one of the following compounds would have these properties?
(Multiple Choice)
4.7/5  (38)
(38)
What is the name of the ionic compound with the formula Na3PO4?
(Multiple Choice)
4.8/5  (35)
(35)
The ionic compound magnesium hydroxide can be found in antacid tablets.What is the formula of magnesium hydroxide?
(Multiple Choice)
4.7/5  (37)
(37)
As a rule,ionic compounds tend to have lower melting and boiling points than covalent compounds consisting of small molecules.
(True/False)
4.7/5  (37)
(37)
Showing 81 - 93 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)