Exam 3: Structure and Properties of Ionic and Covalent Compounds
Exam 1: Chemistry - Methods and Measurement104 Questions
Exam 2: The Structure of the Atom and the Periodic Table115 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds93 Questions
Exam 4: Calculations, chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter: Gases, liquids, and Solids76 Questions
Exam 6: Solutions69 Questions
Exam 7: Energy,rate and Equilibrium67 Questions
Exam 8: Acids and Bases67 Questions
Exam 9: The Nucleus, radioactivity, and Nuclear Medicine86 Questions
Exam 10: An Introduction to Organic Chemistry104 Questions
Exam 11: The Unsaturated Hydrocarbons: Alkenes, alkynes, and Aromatics74 Questions
Exam 12: Alcohols, phenols, thiols, and Ethers69 Questions
Exam 13: Aldehydes and Ketones63 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives83 Questions
Exam 15: Amines and Amides82 Questions
Exam 16: Carbohydrates81 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems78 Questions
Exam 18: Protein Structure and Function75 Questions
Exam 19: Enzymes74 Questions
Exam 20: Introduction to Molecular Genetics85 Questions
Exam 21: Carbohydrate Metabolism76 Questions
Exam 22: Aerobic Respiration and Energy Production75 Questions
Exam 23: Fatty Acid Metabolism75 Questions
Select questions type
In the molecule BeF2,the beryllium atom is an exception to the octet rule.
(True/False)
4.7/5  (50)
(50)
How many bonding electrons are present in the Lewis structure for the bicarbonate ion,shown below? 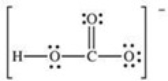
(Multiple Choice)
4.7/5  (36)
(36)
Baking soda consists of the ionic compound sodium bicarbonate.What is the formula of this compound?
(Multiple Choice)
4.8/5  (32)
(32)
In the molecule AX2,the central atom A has two lone pairs of electrons in addition to the two bond pairs in the A-X bonds.What is the shape of this molecule?
(Multiple Choice)
4.8/5  (47)
(47)
How many dots are present in the Lewis symbol for the fluorine atom?
(Multiple Choice)
4.9/5  (41)
(41)
What does it mean if an atom is said to have a high electronegativity?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following Lewis structures has a possible resonance structure? 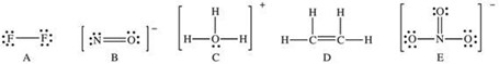
(Multiple Choice)
4.9/5  (31)
(31)
The drug "lithium" is often prescribed to treat mental illness.It is not the element lithium,but the ionic compound Li2CO3,that is actually administered.What is the name of this compound?
(Multiple Choice)
4.8/5  (34)
(34)
What is the formula of the ionic compound formed when ions of calcium and nitrogen combine?
(Multiple Choice)
4.9/5  (37)
(37)
What is wrong with the Lewis structure shown for sulfur trioxide,SO3? 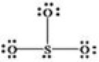
(Multiple Choice)
4.9/5  (29)
(29)
Assuming reactions between the following pairs of elements,which pair is most likely to form a covalent compound?
(Multiple Choice)
4.9/5  (23)
(23)
There are three atoms of iodine represented in the formula NaIO3.
(True/False)
4.9/5  (31)
(31)
Showing 21 - 40 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)