Exam 3: Structure and Properties of Ionic and Covalent Compounds
Exam 1: Chemistry - Methods and Measurement104 Questions
Exam 2: The Structure of the Atom and the Periodic Table115 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds93 Questions
Exam 4: Calculations, chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter: Gases, liquids, and Solids76 Questions
Exam 6: Solutions69 Questions
Exam 7: Energy,rate and Equilibrium67 Questions
Exam 8: Acids and Bases67 Questions
Exam 9: The Nucleus, radioactivity, and Nuclear Medicine86 Questions
Exam 10: An Introduction to Organic Chemistry104 Questions
Exam 11: The Unsaturated Hydrocarbons: Alkenes, alkynes, and Aromatics74 Questions
Exam 12: Alcohols, phenols, thiols, and Ethers69 Questions
Exam 13: Aldehydes and Ketones63 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives83 Questions
Exam 15: Amines and Amides82 Questions
Exam 16: Carbohydrates81 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems78 Questions
Exam 18: Protein Structure and Function75 Questions
Exam 19: Enzymes74 Questions
Exam 20: Introduction to Molecular Genetics85 Questions
Exam 21: Carbohydrate Metabolism76 Questions
Exam 22: Aerobic Respiration and Energy Production75 Questions
Exam 23: Fatty Acid Metabolism75 Questions
Select questions type
Which compound contains a central atom with an exception to the octet rule known as an expanded octet?
(Multiple Choice)
4.9/5  (39)
(39)
The hydrogen bonds between water and ammonia in a solution are intermolecular forces.
(True/False)
4.8/5  (38)
(38)
The existence of resonance makes a molecule less stable than would otherwise be the case.
(True/False)
4.8/5  (41)
(41)
Resonance occurs when two or more different,valid Lewis structures can be drawn for a molecule.
(True/False)
4.9/5  (28)
(28)
Vehicle airbags inflate when the ionic compound sodium azide rapidly decomposes to the elements sodium and nitrogen.If the azide ion is a polyatomic ion with the formula N3−,what is the formula of sodium azide?
(Multiple Choice)
4.9/5  (33)
(33)
Which statement about general bonding characteristics is FALSE?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following is an essential feature of ionic bonding?
(Multiple Choice)
4.9/5  (38)
(38)
Assuming reactions between the following pairs of elements,which pair is most likely to form an ionic compound?
(Multiple Choice)
4.8/5  (40)
(40)
Formaldehyde is a covalent compound used as a preservative for biological specimens.Which statement concerning the Lewis structure of formaldehyde,shown below,is FALSE? 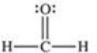
(Multiple Choice)
4.7/5  (41)
(41)
Which combination of atoms is most likely to form an ionic compound if they are allowed to react with each other?
(Multiple Choice)
4.8/5  (41)
(41)
What is the formula of the ionic compound sodium carbonate?
(Multiple Choice)
4.8/5  (35)
(35)
In determining properties such as solubility,melting point,and boiling point,intramolecular forces are more important than intermolecular forces.
(True/False)
5.0/5  (40)
(40)
The ionic compound iron(II)sulfate is used in iron-containing supplements.What does the (II)in the name of this compound specifically indicate?
(Multiple Choice)
4.7/5  (42)
(42)
In Lewis symbols,the chemical symbol of an element represents both the nucleus and the lower energy (nonvalence)electrons.
(True/False)
4.8/5  (26)
(26)
Showing 61 - 80 of 93
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)