Exam 9: Nucleophilic Substitution and Beta-Elimination
Exam 1: Covalent Bonding and Shapes of Molecules118 Questions
Exam 2: Alkanes and Cycloalkanes101 Questions
Exam 3: Stereoisomerism and Chirality95 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties88 Questions
Exam 6: Reactions of Alkenes97 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions75 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination104 Questions
Exam 10: Alcohols102 Questions
Exam 11: Ethers, Epoxides, and Sulfides102 Questions
Exam 12: Infrared Spectroscopy74 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy99 Questions
Exam 14: Mass Spectrometry75 Questions
Exam 15: An Introduction to Organometallic Compounds73 Questions
Exam 16: Aldehydes and Ketones122 Questions
Exam 17: Carboxylic Acids76 Questions
Exam 18: Functional Derivatives of Carboxylic Acids117 Questions
Exam 19: Enolate Anions and Enamines96 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions75 Questions
Exam 21: Benzene and the Concept of Aromaticity77 Questions
Exam 22: Reactions of Benzene and Its Derivatives109 Questions
Exam 23: Amines92 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation84 Questions
Exam 25: Carbohydrates69 Questions
Exam 26: Lipids65 Questions
Exam 27: Amino Acids and Proteins77 Questions
Exam 28: Nucleic Acids58 Questions
Exam 29: Organic Polymer Chemistry68 Questions
Select questions type
Which of the following alkyl halides undergoes the fastest solvolysis reaction with ethanol, CH3CH2OH?
(Multiple Choice)
4.8/5  (40)
(40)
Draw all of the chloroalkanes that undergo base-promoted dehydrochlorination to form the following alkene? 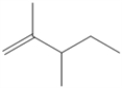
(Essay)
4.7/5  (43)
(43)
Which of the following solvents is the best choice for the reaction of 1-chlorohexane with sodium bromide?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following alkyl halides undergoes the fastest solvolysis reaction with methanol, CH3OH?
(Multiple Choice)
4.9/5  (25)
(25)
Which of the following energy diagrams represents the course of an exothermic SN1 reaction? 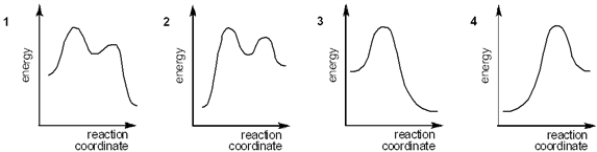
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following statements is not true regarding the SN2 reaction of (R)-2-bromobutane with sodium cyanide?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following is not a characteristic of SN1 reactions?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following alkyl halides undergoes the fastest solvolysis reaction with formic acid, HCOOH?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium azide, NaN3?
(Multiple Choice)
4.7/5  (42)
(42)
In which of the following solvents would the reaction of 1-bromobutane with sodium azide, NaN3, proceed the fastest?
(Multiple Choice)
4.7/5  (32)
(32)
What is the major elimination product obtained from the following reaction? 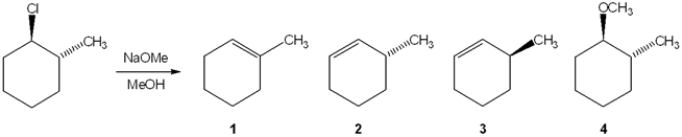
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following represents the transition state of the rate-determining step in the SN1 reaction between tert-butyl chloride and water? 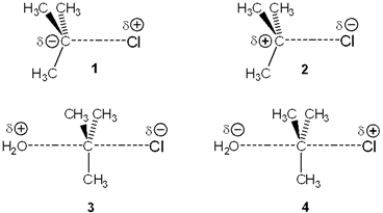
(Multiple Choice)
4.8/5  (40)
(40)
In the case of a unimolecular nucleophilic substitution reaction, the reaction rate completely depends on the concentration of the _____.
(Short Answer)
4.8/5  (33)
(33)
What is(are) the major organic product(s) obtained from the following substitution reaction? 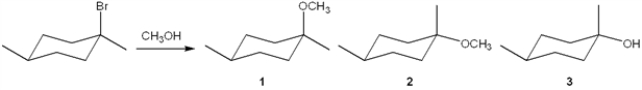
(Multiple Choice)
4.8/5  (36)
(36)
The reaction of 1-bromopropane with sodium iodide gives 1-iodopropane. What is the effect of doubling the concentration of NaI on the rate of the reaction?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following most favors elimination rather substitution in a reaction with sodium methoxide?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following compounds is the most nucleophilic in polar protic solvents?
(Multiple Choice)
4.8/5  (50)
(50)
Consider the reaction below to answer the following question: 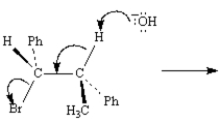 The product of this reaction is
The product of this reaction is 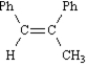
(True/False)
4.9/5  (36)
(36)
Which of the following is the best leaving group in an SN2 reaction?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 41 - 60 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)