Exam 9: Nucleophilic Substitution and Beta-Elimination
Exam 1: Covalent Bonding and Shapes of Molecules118 Questions
Exam 2: Alkanes and Cycloalkanes101 Questions
Exam 3: Stereoisomerism and Chirality95 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties88 Questions
Exam 6: Reactions of Alkenes97 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions75 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination104 Questions
Exam 10: Alcohols102 Questions
Exam 11: Ethers, Epoxides, and Sulfides102 Questions
Exam 12: Infrared Spectroscopy74 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy99 Questions
Exam 14: Mass Spectrometry75 Questions
Exam 15: An Introduction to Organometallic Compounds73 Questions
Exam 16: Aldehydes and Ketones122 Questions
Exam 17: Carboxylic Acids76 Questions
Exam 18: Functional Derivatives of Carboxylic Acids117 Questions
Exam 19: Enolate Anions and Enamines96 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions75 Questions
Exam 21: Benzene and the Concept of Aromaticity77 Questions
Exam 22: Reactions of Benzene and Its Derivatives109 Questions
Exam 23: Amines92 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation84 Questions
Exam 25: Carbohydrates69 Questions
Exam 26: Lipids65 Questions
Exam 27: Amino Acids and Proteins77 Questions
Exam 28: Nucleic Acids58 Questions
Exam 29: Organic Polymer Chemistry68 Questions
Select questions type
The reaction of methyl iodide with sodium azide, NaN3, proceeds by an SN2 mechanism. What is the effect of doubling the concentration of NaN3 on the rate of the reaction?
(Multiple Choice)
4.9/5  (37)
(37)
Why is cyclohexylamine more reactive than aniline when the former reacts with methyl bromide?
(Essay)
4.8/5  (37)
(37)
Which of the following statements is true regarding the reactivity of 1 and 2 with potassium tert-butoxide? 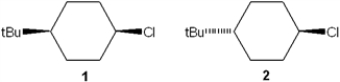
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following statements related to SN1 reactions is not true?
(Multiple Choice)
4.8/5  (35)
(35)
What is the major organic product obtained from the following reaction? 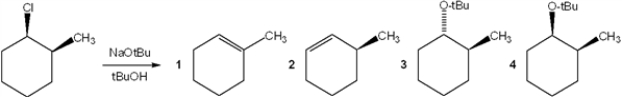
(Multiple Choice)
4.8/5  (40)
(40)
What is the major organic product obtained from the following reaction? 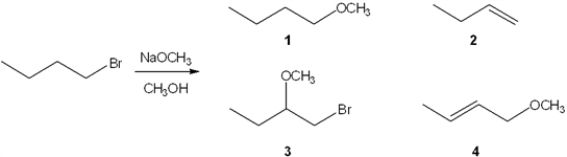
(Multiple Choice)
4.8/5  (27)
(27)
What is the equation for the rate of formation of tert-butyl alcohol from the reaction of tert-butyl bromide (t-BuBr) with water by an SN1 mechanism?
(Multiple Choice)
4.8/5  (32)
(32)
The reaction of tert-butyl chloride, (CH3)3CCl, with water in an inert solvent gives tert-butyl alcohol, (CH3)3COH. What is the effect of doubling the concentration of water on the rate of the reaction?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium cyanide, NaCN?
(Multiple Choice)
4.9/5  (41)
(41)
What is the major organic product obtained from the following reaction? 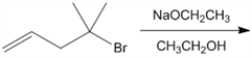
(Essay)
4.9/5  (32)
(32)
Which of the following is the best set of conditions for the preparation of tert-butyl methyl ether?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following energy diagrams represents the course of an exothermic E2 reaction? 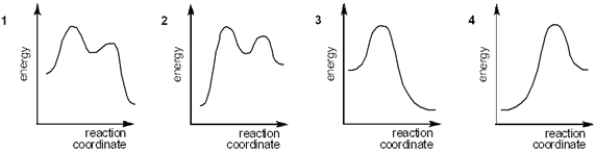
(Multiple Choice)
4.9/5  (30)
(30)
Provide a neatly drawn mechanism for the following reaction, including curved arrows to show the movement of pairs of electrons and the structure of reactive intermediates. 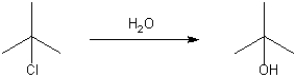
(Essay)
4.9/5  (43)
(43)
What is the major organic product obtained from the following reaction? 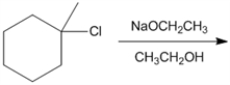
(Essay)
4.9/5  (35)
(35)
Draw all of the chloroalkanes that undergo base-promoted dehydrochlorination to form the following alkene? 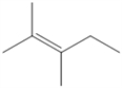
(Essay)
4.8/5  (33)
(33)
What is the major organic product obtained from the following reaction? 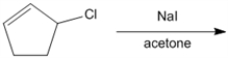
(Essay)
4.8/5  (29)
(29)
Which of the following reactions corresponds to a substitution?
(Multiple Choice)
4.8/5  (24)
(24)
The following reaction would have a faster rate in a higher polarity solvent than one of low polarity. 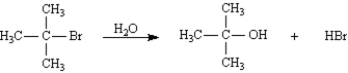
(True/False)
4.9/5  (31)
(31)
Showing 61 - 80 of 104
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)