Exam 1: Covalent Bonding and Shapes of Molecules
Exam 1: Covalent Bonding and Shapes of Molecules118 Questions
Exam 2: Alkanes and Cycloalkanes101 Questions
Exam 3: Stereoisomerism and Chirality95 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties88 Questions
Exam 6: Reactions of Alkenes97 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions75 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination104 Questions
Exam 10: Alcohols102 Questions
Exam 11: Ethers, Epoxides, and Sulfides102 Questions
Exam 12: Infrared Spectroscopy74 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy99 Questions
Exam 14: Mass Spectrometry75 Questions
Exam 15: An Introduction to Organometallic Compounds73 Questions
Exam 16: Aldehydes and Ketones122 Questions
Exam 17: Carboxylic Acids76 Questions
Exam 18: Functional Derivatives of Carboxylic Acids117 Questions
Exam 19: Enolate Anions and Enamines96 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions75 Questions
Exam 21: Benzene and the Concept of Aromaticity77 Questions
Exam 22: Reactions of Benzene and Its Derivatives109 Questions
Exam 23: Amines92 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation84 Questions
Exam 25: Carbohydrates69 Questions
Exam 26: Lipids65 Questions
Exam 27: Amino Acids and Proteins77 Questions
Exam 28: Nucleic Acids58 Questions
Exam 29: Organic Polymer Chemistry68 Questions
Select questions type
Which of the following elements has the highest electronegativity?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
C
Which of the following statements is not true about the carbonate anion, CO32−?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
C
The maximum number of electrons that a molecular orbital can contain is four.
Free
(True/False)
4.7/5  (35)
(35)
Correct Answer:
False
Which atomic orbitals overlap to form the carbon-hydrogen σ bonding molecular orbitals of ethyne, HC≡CH?
(Multiple Choice)
4.8/5  (48)
(48)
Different compounds with the same molecular formula are called __________.
(Short Answer)
4.9/5  (34)
(34)
How many electrons are there in the valence shell of the oxygen atom of water?
(Multiple Choice)
4.7/5  (28)
(28)
Which of the following is a primary (1°) alcohol? | || 1 2 3 4
(Multiple Choice)
4.9/5  (41)
(41)
The curved arrows in the resonance structure for the acetate ion shown below 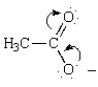 indicate the following alternative resonance structure for the acetate ion.
indicate the following alternative resonance structure for the acetate ion. 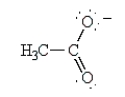
(True/False)
4.8/5  (42)
(42)
Draw bond-line structures of all of the secondary (2°) amines that have the formula C4H9N.
(Essay)
4.8/5  (35)
(35)
What is the ground-state electronic configuration of a fluoride anion (fluorine: atomic number 9)?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following species has an atom that has an unfilled valence shell of electrons?
(Multiple Choice)
4.8/5  (31)
(31)
The most electronegative elements in the periodic table are generally found toward the right in a horizontal row and toward the top in a column.
(True/False)
4.8/5  (42)
(42)
Rank the following in order of decreasing importance as a contributing resonance structure to the molecular structure of acetone, CH3COCH3 (more important > less important) 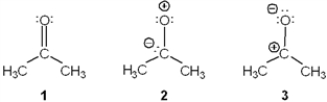
(Multiple Choice)
4.8/5  (42)
(42)
Showing 1 - 20 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)