Exam 4: Acids and Bases
Exam 1: Covalent Bonding and Shapes of Molecules118 Questions
Exam 2: Alkanes and Cycloalkanes101 Questions
Exam 3: Stereoisomerism and Chirality95 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties88 Questions
Exam 6: Reactions of Alkenes97 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions75 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination104 Questions
Exam 10: Alcohols102 Questions
Exam 11: Ethers, Epoxides, and Sulfides102 Questions
Exam 12: Infrared Spectroscopy74 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy99 Questions
Exam 14: Mass Spectrometry75 Questions
Exam 15: An Introduction to Organometallic Compounds73 Questions
Exam 16: Aldehydes and Ketones122 Questions
Exam 17: Carboxylic Acids76 Questions
Exam 18: Functional Derivatives of Carboxylic Acids117 Questions
Exam 19: Enolate Anions and Enamines96 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions75 Questions
Exam 21: Benzene and the Concept of Aromaticity77 Questions
Exam 22: Reactions of Benzene and Its Derivatives109 Questions
Exam 23: Amines92 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation84 Questions
Exam 25: Carbohydrates69 Questions
Exam 26: Lipids65 Questions
Exam 27: Amino Acids and Proteins77 Questions
Exam 28: Nucleic Acids58 Questions
Exam 29: Organic Polymer Chemistry68 Questions
Select questions type
Complete the equation below for the protonation of cyclohexene with HCl. Show the movement of pairs of electrons with curved arrows and provide the structures of the conjugate acid and conjugate base. 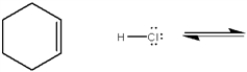
(Essay)
4.8/5  (54)
(54)
Complete the equation below for the protonation of 2-butene with HBr. Show the movement of pairs of electrons with curved arrows and provide the structures of the conjugate acid and conjugate base. 
(Essay)
4.8/5  (41)
(41)
What is the value of the equilibrium constant, Keq, for the following reaction? 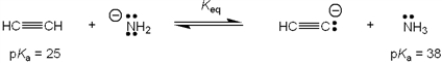
(Multiple Choice)
5.0/5  (36)
(36)
The following correctly shows the electron flow for the given reaction. 
(True/False)
5.0/5  (33)
(33)
The pKa of acetic acid, CH3COOH, is 4.76. What is the value of the equilibrium constant Keq, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M]
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following is a feature of a Brønsted-Lowry base?
(Multiple Choice)
4.8/5  (34)
(34)
Which species is the conjugate acid in the following acid-base reaction? 
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following has a pKa value of approximately 25?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following is a definition of the activation energy of a reaction?
(Multiple Choice)
5.0/5  (37)
(37)
Provide the equation that relates the acid dissociation constant, Ka, to the pKa of an acid.
(Short Answer)
4.9/5  (42)
(42)
What is the value of the equilibrium constant for the following equilibrium?
(Essay)
4.8/5  (34)
(34)
Provide a brief explanation, based on features of the molecules, for the following trend in pKa values?
CH3CH2OH > CF3CH2OH
ethanol 2,2,2-trifluoromethanol
15.9 12.4
(Essay)
4.8/5  (33)
(33)
Which of the following is a feature of a Brønsted-Lowry acid?
(Multiple Choice)
4.9/5  (40)
(40)
Use curved arrows to show the movement of pairs of electrons in the following acid-base reaction and show the structures of the conjugate acid and conjugate base. 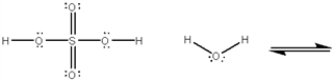
(Essay)
4.9/5  (37)
(37)
Consider the following reaction coordinate diagram. 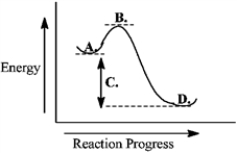 This reaction is endergonic.
This reaction is endergonic.
(True/False)
4.9/5  (36)
(36)
Consider the following reaction coordinate diagram. 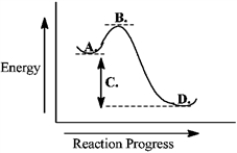 The free energy of activation is represented by the letter C.
The free energy of activation is represented by the letter C.
(True/False)
4.9/5  (31)
(31)
Showing 41 - 60 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)