Exam 4: Acids and Bases
Exam 1: Covalent Bonding and Shapes of Molecules118 Questions
Exam 2: Alkanes and Cycloalkanes101 Questions
Exam 3: Stereoisomerism and Chirality95 Questions
Exam 4: Acids and Bases94 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties88 Questions
Exam 6: Reactions of Alkenes97 Questions
Exam 7: Alkynes100 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions75 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination104 Questions
Exam 10: Alcohols102 Questions
Exam 11: Ethers, Epoxides, and Sulfides102 Questions
Exam 12: Infrared Spectroscopy74 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy99 Questions
Exam 14: Mass Spectrometry75 Questions
Exam 15: An Introduction to Organometallic Compounds73 Questions
Exam 16: Aldehydes and Ketones122 Questions
Exam 17: Carboxylic Acids76 Questions
Exam 18: Functional Derivatives of Carboxylic Acids117 Questions
Exam 19: Enolate Anions and Enamines96 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions75 Questions
Exam 21: Benzene and the Concept of Aromaticity77 Questions
Exam 22: Reactions of Benzene and Its Derivatives109 Questions
Exam 23: Amines92 Questions
Exam 24: Catalytic Carbon-Carbon Bond Formation84 Questions
Exam 25: Carbohydrates69 Questions
Exam 26: Lipids65 Questions
Exam 27: Amino Acids and Proteins77 Questions
Exam 28: Nucleic Acids58 Questions
Exam 29: Organic Polymer Chemistry68 Questions
Select questions type
Provide the equation that relates the equilibrium constant, Keq, to the acid dissociation constant, Ka, for the following equilibrium.
(Short Answer)
4.7/5  (37)
(37)
Which of the following is a Lewis acid but not a Brønsted-Lowry acid?
(Multiple Choice)
4.9/5  (39)
(39)
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product. 
(Essay)
4.8/5  (43)
(43)
Which atom in the following structure is preferentially protonated by a strong acid? 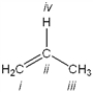
(Multiple Choice)
4.8/5  (41)
(41)
Which sets of curved arrows accounts for the protonation of propene with HI? 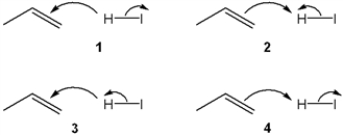
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following is present in the highest concentration upon dissolution of acetic acid in water?
(Multiple Choice)
4.9/5  (50)
(50)
Which of the following is the correct order of decreasing basicity (stronger base > weaker base)?
(Multiple Choice)
4.8/5  (36)
(36)
Use curved arrows to show the movement of pairs of electrons in the following reaction between a Lewis acid and a Lewis base, and show the structure of the product. 
(Essay)
4.8/5  (31)
(31)
Match the types of reactions with the most appropriate relationships.
-ΔHproduct > ΔHreactant
(Multiple Choice)
4.9/5  (42)
(42)
The following is generic depiction of a reaction using the curve arrow formalism. 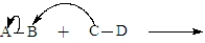 In this reaction electrons move from C to B and A will have a positive charge in the product.
In this reaction electrons move from C to B and A will have a positive charge in the product.
(True/False)
4.8/5  (42)
(42)
Showing 81 - 94 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)