Exam 14: Temperature and Heat
Exam 1: Introduction73 Questions
Exam 2: Motion, Forces, and Newtons Laws61 Questions
Exam 3: Forces and Motion in One Dimension77 Questions
Exam 4: Forces and Motion in Two and Three Dimensions86 Questions
Exam 5: Circular Motion and Gravitation55 Questions
Exam 6: Work and Energy89 Questions
Exam 7: Momentum, Impulse, and Collisions86 Questions
Exam 8: Rotational Motion76 Questions
Exam 9: Energy and Momentum of Rotational Motion52 Questions
Exam 10: Fluids74 Questions
Exam 11: Harmonic Motion and Elasticity71 Questions
Exam 12: Waves58 Questions
Exam 13: Sound69 Questions
Exam 14: Temperature and Heat115 Questions
Exam 15: Gases and Kinetic Theory63 Questions
Exam 16: Thermodynamics68 Questions
Exam 17: Electric Forces and Fields80 Questions
Exam 18: Electric Potential91 Questions
Exam 19: Electric Currents and Circuits115 Questions
Exam 20: Magnetic Fields and Forces77 Questions
Exam 21: Magnetic Induction70 Questions
Exam 22: Alternating-Current Circuits and Machines64 Questions
Exam 23: Electromagnetic Waves53 Questions
Exam 24: Geometrical Optics127 Questions
Exam 25: Wave Optics83 Questions
Exam 26: Applications of Optics55 Questions
Exam 27: Relativity77 Questions
Exam 28: Quantum Theory63 Questions
Exam 29: Atomic Theory63 Questions
Exam 30: Nuclear Physics70 Questions
Exam 31: Physics in the 21st Century35 Questions
Select questions type
Pennies used to be made of copper, but now they are made of copper-coated zinc. If one were to do a precise calorimetry experiment to determine the specific heat of the new pennies, what would be the result to be?
(Multiple Choice)
5.0/5  (34)
(34)
Carbon dioxide and water molecules in the atmosphere will absorb:
(Multiple Choice)
4.8/5  (36)
(36)
A 0.2-kg aluminum plate, initially at 20 C, slides down a 10-m long surface, inclined at a 30 angle to the horizontal. The force of kinetic friction exactly balances the component of gravity down the plane so that the plate, once started, glides down at constant velocity. If 90% of the mechanical energy of the system is absorbed by the aluminum, what is its temperature increase at the bottom of the incline? (Specific heat for aluminum is 900 J/kg · C.)
(Multiple Choice)
4.8/5  (43)
(43)
In the text there is a table of coefficients of linear ( 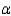 ) and volume (
) and volume ( 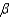 ) expansions due to temperature changes. For the solid materials in this list, there is a relationship between
) expansions due to temperature changes. For the solid materials in this list, there is a relationship between 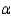 and
and 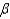 which allows you to find the value of
which allows you to find the value of 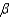 if you know
if you know 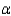 . Which of the following best describes that relationship?
. Which of the following best describes that relationship?
(Multiple Choice)
4.8/5  (38)
(38)
A machine gear consists of 0.40 kg of iron and 0.16 kg of copper. How much total heat is generated in the part if its temperature increases by 35 C? (Specific heats of iron and copper are 450 and 390 J/kg · C, respectively.)
(Multiple Choice)
4.9/5  (37)
(37)
A steel plate has a hole drilled through it. The plate is put into a furnace and heated. What happens to the size of the inside diameter of a hole as its temperature increases?
(Multiple Choice)
4.8/5  (31)
(31)
A plot of the temperature versus the energy per kg added to a piece of ice as it goes from below freezing at -10 C to becoming steam at 110 C consists of straight lines, some horizontal and some with an upward slope. What do the upward slopes represent?
(Multiple Choice)
4.9/5  (34)
(34)
Which best describes a system made up of ice, water, and steam existing together?
(Multiple Choice)
4.8/5  (28)
(28)
How much heat energy is required to vaporize a 1.0-g ice cube at 0 C? The heat of fusion of ice is 80 cal/g. The heat of vaporization of water is 540 cal/g, and cwater = 1.00 cal/g· C.
(Multiple Choice)
4.9/5  (35)
(35)
Carbon dioxide forms into a solid (dry ice) at approximately -157 F. What temperature in degrees Celsius does this correspond to?
(Multiple Choice)
4.9/5  (42)
(42)
Inside a house, stepping on a tile floor barefooted may feel almost cold, but stepping on carpet in an adjacent room feels comfortably warm. Why is this?
(Multiple Choice)
4.8/5  (27)
(27)
At high noon, the Sun delivers 900 W to each square meter of a blacktop road. What is the equilibrium temperature of the asphalt, assuming its emissivity e = 1? ( = 5.67*10-8 W/m2*K4) .
(Multiple Choice)
4.8/5  (28)
(28)
The surface of the Sun has a temperature of about 5800 K. If the radius of the Sun is 7*108 m, determine the power output of the sun. (Take e = 1, and = 5.67 *10-8 W/m2 ·K4).
(Multiple Choice)
4.9/5  (29)
(29)
A puddle holds 150 g of water. If 0.75 g of water evaporates from the surface, what is the approximate temperature change of the remaining water? (Lv = 540 cal/g)
(Multiple Choice)
4.8/5  (45)
(45)
When heating 2 kg of ice from 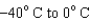 , 2 kg of water from
, 2 kg of water from 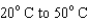 , and 2 kg of steam from
, and 2 kg of steam from 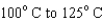 , which requires the most energy?
, which requires the most energy?
(Multiple Choice)
4.8/5  (40)
(40)
Carly places one end of a steel bar in a Bunsen flame and the other end in an ice cube. By what factor is the rate of heat flow changed when the bar's cross-sectional area is tripled?
(Multiple Choice)
4.8/5  (33)
(33)
What happens to a given volume of water when heated from 0 C to 4 C?
(Multiple Choice)
4.9/5  (38)
(38)
A 3.00 g lead bullet is traveling at a speed of 300 m/s when it embeds in a wood post. If we assume that half of the resultant heat energy generated remains with the bullet, what is the increase in temperature of the embedded bullet? (specific heat of lead = 0.0305 kcal/kg· C, 1 kcal = 4186 J)
(Multiple Choice)
4.9/5  (35)
(35)
A solar heated house loses about 7.4 *107 cal through its outer surfaces on a typical 24-hour winter day. What mass of storage rock is needed to provide this amount of heat if it is brought up to initial temperature of 62 C by the solar collectors, and the house is maintained at 20 C? (Specific heat of rock is 0.21 cal/g · C.)
(Multiple Choice)
5.0/5  (43)
(43)
Showing 21 - 40 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)