Exam 14: Temperature and Heat
Exam 1: Introduction73 Questions
Exam 2: Motion, Forces, and Newtons Laws61 Questions
Exam 3: Forces and Motion in One Dimension77 Questions
Exam 4: Forces and Motion in Two and Three Dimensions86 Questions
Exam 5: Circular Motion and Gravitation55 Questions
Exam 6: Work and Energy89 Questions
Exam 7: Momentum, Impulse, and Collisions86 Questions
Exam 8: Rotational Motion76 Questions
Exam 9: Energy and Momentum of Rotational Motion52 Questions
Exam 10: Fluids74 Questions
Exam 11: Harmonic Motion and Elasticity71 Questions
Exam 12: Waves58 Questions
Exam 13: Sound69 Questions
Exam 14: Temperature and Heat115 Questions
Exam 15: Gases and Kinetic Theory63 Questions
Exam 16: Thermodynamics68 Questions
Exam 17: Electric Forces and Fields80 Questions
Exam 18: Electric Potential91 Questions
Exam 19: Electric Currents and Circuits115 Questions
Exam 20: Magnetic Fields and Forces77 Questions
Exam 21: Magnetic Induction70 Questions
Exam 22: Alternating-Current Circuits and Machines64 Questions
Exam 23: Electromagnetic Waves53 Questions
Exam 24: Geometrical Optics127 Questions
Exam 25: Wave Optics83 Questions
Exam 26: Applications of Optics55 Questions
Exam 27: Relativity77 Questions
Exam 28: Quantum Theory63 Questions
Exam 29: Atomic Theory63 Questions
Exam 30: Nuclear Physics70 Questions
Exam 31: Physics in the 21st Century35 Questions
Select questions type
If the surface temperature of the Earth is 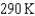 and that of the Sun is 6000 K, assuming the same emissivities, what is the ratio of the energy radiated per square meter of the Earth to that of the Sun?
and that of the Sun is 6000 K, assuming the same emissivities, what is the ratio of the energy radiated per square meter of the Earth to that of the Sun?
(Multiple Choice)
4.8/5  (27)
(27)
The thermal conductivity of aluminum is 238 W / m · C and of copper is 397 W / m · C. A rod of each material is used as a heat conductor. If the rods have the same geometry and are used between the same temperature differences for the same time interval, what is the ratio of the heat transferred by the aluminum to the heat transferred by the copper?
(Multiple Choice)
4.9/5  (37)
(37)
Suppose working outside on a hot day someone generate 450 J of heat each second. If this energy is compensated for by the evaporation of sweat, how much water would be lost in an hour? The latent heat of fusion for water is 334 kJ/kg and for vaporization is 2,200 kJ/kg.
(Multiple Choice)
4.8/5  (32)
(32)
The filament temperature of a light bulb is 2000 K when the bulb delivers 50 W of power. If its emissivity remains constant, what power is delivered when the filament temperature is 2500 K?
(Multiple Choice)
4.9/5  (34)
(34)
A windowpane is half a centimeter thick and has an area of 2.0 m2. The temperature difference between the inside and outside surfaces of the pane is 15 C. What is the rate of heat flow through this window? (Thermal conductivity for this glass is 0.84 W / m · C.)
(Multiple Choice)
4.8/5  (33)
(33)
As a copper wire is heated, its length increases by 0.200%. What is the change of the temperature of the wire? ( Cu = 16.6 *10 -6/C )
(Multiple Choice)
4.9/5  (37)
(37)
At what temperature is the same numerical value obtained in Celsius and Fahrenheit?
(Multiple Choice)
4.7/5  (29)
(29)
When a wool blanket is used to keep warm, what is the primary insulating material?
(Multiple Choice)
5.0/5  (44)
(44)
At room temperature, the coefficient of linear expansion for Pyrex glass is _____ that for ordinary glass.
(Multiple Choice)
4.8/5  (37)
(37)
I take 2.0 kg of ice and dump it into 1.0 kg of water and, when equilibrium is reached, I have 3.0 kg of ice at 0 C. The water was originally at 0 C. The specific heat of water = 1.00 kcal/kg· C, the specific heat of ice = 0.50 kcal/kg· C, and the latent heat of fusion of water is 80 kcal/kg. The original temperature of the ice was:
(Multiple Choice)
4.8/5  (35)
(35)
A rectangular steel plate with dimensions of 60 cm *25 cm is heated from 20 C to 150 C. What is its change in area? (Coefficient of linear expansion for steel is 11 *10 - 6/ C.)
(Multiple Choice)
4.8/5  (35)
(35)
Calories of the food type are equal to which of the following?
(Multiple Choice)
4.9/5  (33)
(33)
Arrange the following units, Calorie, calorie, and Joule from smallest to largest.
(Multiple Choice)
4.8/5  (35)
(35)
A 2.00-kg copper rod is 40.00 cm long at 23°C. If 50,000 J are transferred to the rod by heat, what is its change in length? (ccopper = 387 J/kg·°C and copper = 17 *10-6/°C)
(Multiple Choice)
4.9/5  (42)
(42)
Between 0 C and 4 C, the volume coefficient of expansion for water:
(Multiple Choice)
4.7/5  (38)
(38)
Dmitri places one end of a copper rod in a heat reservoir and the other end in a heat sink. By what factor is the rate of heat flow changed when the temperature difference between the reservoir and sink is doubled?
(Multiple Choice)
4.9/5  (37)
(37)
An object at 27 C has its temperature increased to 47 C. The power then radiated by this object increases by how many percent?
(Multiple Choice)
4.8/5  (36)
(36)
At 20 C an aluminum ring has an inner diameter of 5.000 cm, and a brass rod has a diameter of 5.020 cm. Keeping the brass rod at 20°C, which of the following temperatures of the ring will allow the ring to just slip over the brass rod? ( Al = 2.4 *10 - 5 / C, brass = 1.9 *10 - 5/ C )
(Multiple Choice)
4.8/5  (32)
(32)
Marc attaches a falling 250-kg object with a rope through a pulley to a paddle wheel shaft. He places the system in a well-insulated tank holding 12 kg of water. When the object falls, it causes the paddle wheel to rotate and churn the water. If the object falls a vertical distance of 100 m at constant speed, what is the temperature change of the water? (1 kcal = 4186 J, the specific heat of water is 4186 J/kg · C, and g = 9.8 m/s2)
(Multiple Choice)
4.9/5  (33)
(33)
Showing 81 - 100 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)