Exam 3: Ionic Compounds
Exam 1: Matter and Measurement87 Questions
Exam 2: Atoms and the Periodic Table95 Questions
Exam 3: Ionic Compounds100 Questions
Exam 4: Covalent Compounds101 Questions
Exam 5: Chemical Reactions98 Questions
Exam 6: Energy Changes,reaction Rates,and Equilibrium102 Questions
Exam 7: Gases,liquids,and Solids98 Questions
Exam 8: Solutions98 Questions
Exam 9: Acids and Bases108 Questions
Exam 10: Nuclear Chemistry93 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups123 Questions
Exam 12: Alkanes104 Questions
Exam 13: Unsaturated Hydrocarbons104 Questions
Exam 14: Organic Compounds That Contain Oxygen,halogen,or Sulfur112 Questions
Exam 15: The Three-Dimensional Shape of Molecules101 Questions
Exam 16: Aldehydes and Ketones114 Questions
Exam 17: Carboxylic Acids,esters,and Amides107 Questions
Exam 18: Amines and Neurotransmitters115 Questions
Exam 19: Lipids115 Questions
Exam 20: Carbohydrates100 Questions
Exam 21: Amino Acids,proteins,and Enzymes98 Questions
Exam 22: Nucleic Acids and Protein Synthesis98 Questions
Exam 23: Metabolism and Energy Production102 Questions
Exam 24: Carbohydrate,lipid,and Protein Metabolism99 Questions
Select questions type
The electron configuration for the sulfide ion is 1s22s22p63s23p64s2.
(True/False)
4.9/5  (43)
(43)
If the main group element has an electron-dot symbol of 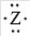 ,the charge of the resulting ion will be -2.
,the charge of the resulting ion will be -2.
(True/False)
4.8/5  (35)
(35)
In crystal of an ionic compound,the cations are surrounded by anions so that the charges of the ions are balanced.
(True/False)
4.9/5  (41)
(41)
________ are negatively charged ions that have ________ electrons than protons.
(Multiple Choice)
4.9/5  (37)
(37)
The ________ rule states that a main group element is especially stable when it possesses eight electrons in its outer shell.
(Short Answer)
4.8/5  (39)
(39)
When forming an ion,an atom with the electron-dot symbol of 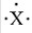 forms an anion with a -3 charge by the loss of three electrons.
forms an anion with a -3 charge by the loss of three electrons.
(True/False)
4.9/5  (24)
(24)
If the main group element has an electron-dot symbol of 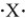 ,the charge of the resulting ion will be +2.
,the charge of the resulting ion will be +2.
(True/False)
4.8/5  (32)
(32)
What is the ion symbol for an atom with twenty (20)protons and eighteen (18)electrons?
(Multiple Choice)
4.8/5  (45)
(45)
Ionic compounds have very high melting points compared to covalent compounds.
(True/False)
4.8/5  (35)
(35)
The chemical formulas of ionic compounds are written with the cation first,and then the anion,with subscripts to show how many of each are needed to have zero net charge.
(True/False)
4.8/5  (42)
(42)
Ionic compounds are composed of positively and negatively charged ions held together by strong electrostatic forces.
(True/False)
4.7/5  (34)
(34)
If atoms with the electron-dot symbols shown below are combined,what is the formula of the ionic compound that is formed? 
(Multiple Choice)
4.9/5  (35)
(35)
Showing 21 - 40 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)