Exam 11: Using Energy
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
An ideal Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C. How much energy does it use to remove 2000 J of heat from the interior of the house?
Free
(Multiple Choice)
5.0/5  (34)
(34)
Correct Answer:
B
An ideal gas undergoes an adiabatic process while doing 25 J of work. What is the change in the internal (thermal)energy of the gas?
Free
(Multiple Choice)
4.7/5  (46)
(46)
Correct Answer:
C
A reversible engine takes in heat at a rate of 555 W and exhausts heat at 482 W.
(a)How much power does it produce?
(b)What is its efficiency?
(c)If operated in reverse as a refrigerator, what would be its performance coefficient (COP)?
Free
(Short Answer)
4.7/5  (30)
(30)
Correct Answer:
(a)73 W (b)13.2% (c)6.6
An ideal Carnot engine has an efficiency of 83.0% and performs 4500 J of work every cycle. How much energy is discharged to the lower temperature reservoir every cycle?
(Multiple Choice)
4.8/5  (36)
(36)
An ideal gas undergoes an isothermal expansion. During this process, its entropy
(Multiple Choice)
4.8/5  (38)
(38)
Two ideal Carnot heat engines have the same efficiency. One operates between 5.0 × 102 K and 3.0 × 102 K, and the other between 4.0 × 102 K and some lower temperature. What is the lower temperature?
(Multiple Choice)
4.7/5  (29)
(29)
A refrigerator has a performance coefficient (COP)of 1.15, and it extracts 7.95 J of heat from the cold reservoir during each cycle.
(a)How much work is done on the gas in each cycle?
(b)How much heat is exhausted into the hot reservoir in each cycle?
(Short Answer)
4.9/5  (39)
(39)
A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 × 106 kg of coal each day, and the heat of combustion of coal is 3.3 × 107 J/kg. The steam that drives the turbines is at a temperature of 300°C, and the exhaust water is at 37°C.
(a)What is the overall efficiency of the plant for generating electric power?
(b)How much thermal energy is exhausted each day?
(c)Using the same heat reservoirs, what is the maximum possible efficiency for a heat engine?
(Short Answer)
4.8/5  (39)
(39)
A certain heat engine extracts 1.30 kJ of heat from a hot temperature reservoir and discharges 0.70 kJ of heat to a cold temperature reservoir. What is the efficiency of this engine?
(Multiple Choice)
4.8/5  (29)
(29)
An ideal Carnot engine operating between a warm reservoir of unknown temperature and a cold reservoir at has an efficiency of What is the temperature of the warm reservoir?
(Multiple Choice)
4.8/5  (42)
(42)
An ideal Carnot engine is operated as a heat pump to heat a room in the winter. The heat pump delivers heat to the room at the rate of 47 kJ per second and maintains the room at a temperature of 293 K when the outside temperature is 237 K. The power requirement to run the heat pump under these operating conditions is closest to
(Multiple Choice)
5.0/5  (30)
(30)
A real (non-Carnot)heat engine, operating between heat reservoirs at temperatures of 450 K and 270 K, performs 3.3 kJ of net work, and rejects 8.2 kJ of heat in a single cycle.
(a)What is the thermal efficiency of this heat engine?
(b)What is the maximum efficiency it could possibly have?
(Short Answer)
4.8/5  (32)
(32)
The figure shows a pV diagram for a cycle of a heat engine for which QH = 59 J. What is the thermal efficiency of the engine? 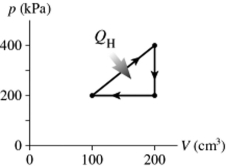
(Multiple Choice)
4.8/5  (37)
(37)
A fluid in an insulated, flexible bottle is heated by a high resistance wire and expands. If of heat is applied to the system and it does of work, how much does the internal (thermal)energy of the fluid change?
(Multiple Choice)
5.0/5  (30)
(30)
A heat engine absorbs 85.6 kJ of heat each cycle and exhausts 61.8 kJ.
(a)What is the efficiency of the engine?
(b)How much work does it do each cycle?
(Short Answer)
4.9/5  (47)
(47)
An inventor tries to sell you his new heat engine that takes in 40 J of heat at 87°C on each cycle, expels 30 J at 27°C, and does 10 J of work. Would it be wise to invest in this engine? Back up your conclusion with numerical calculations.
(Essay)
4.7/5  (38)
(38)
An ideal Carnot refrigerator with a performance coefficient (COP)of 5.0 cools items inside of it to What is the high temperature needed to operate this refrigerator?
(Multiple Choice)
5.0/5  (30)
(30)
The weather outside is frightful. The temperature is -22°F. What is the corresponding temperature in the Celsius scale?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 1 - 20 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)