Exam 11: Using Energy
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
The gas in a perfectly insulated but flexible container does work at a rate of At what rate is the internal (thermal)energy of the gas changing?
(Multiple Choice)
4.9/5  (34)
(34)
A cylinder contains 8.8 moles of ideal gas, initially at a temperature of 126°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of on the gas. The gas is cooled until its temperature has decreased to For the gas The ideal gas constant is R = 8.314 J/mol ? K. For this process, calculate:
(a)the work done by gas
(b)the net change in the internal (thermal)energy of the gas
(c)the heat transferred to the gas.
(Short Answer)
4.8/5  (35)
(35)
An ideal reversible heat pump is taking heat from the outside air at -10.0°C and discharging it into the house at 18.0°C. What is the coefficient of performance of this heat pump?
(Multiple Choice)
4.8/5  (33)
(33)
A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from to The final pressure is What is the change in the internal (thermal)energy of the gas during this process? (R = 8.31 J/mol ? K)
(Multiple Choice)
4.8/5  (29)
(29)
A cylinder contains 13 moles of an ideal gas at a temperature of 300 K. The gas is compressed at constant pressure until the final volume equal 0.70 times the initial volume. The molar heat capacity at constant volume of the gas is 24.0 J/mol ∙ K. What is the change in the internal (thermal)energy of the gas during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (32)
(32)
An expansion process on an ideal diatomic ideal gas for which CV = 5/2 R has a linear path between the initial and final coordinates on a pV diagram. The coordinates of the initial state are: the pressure is the volume is and the temperature is The final pressure is and the final temperature is What is the change in the internal (thermal)energy of the gas, during this process? (R = 8.31 J/mol ? K)
(Multiple Choice)
4.7/5  (37)
(37)
A sealed rigitd tank contains 30 moles of an ideal gas, at an initial temperature of The pressure of the gas is increased until the final pressure equals 1.40 times the initial pressure. The heat capacity at constant pressure of the gas is What is the change in the internal (thermal)energy of the gas during this process? (R = 8.31 J/mol ? K)
(Multiple Choice)
4.8/5  (30)
(30)
During each cycle, a refrigerator removes 20.0 kJ of heat from the freezing compartment and ejects 24.0 kJ into a room.
(a)How much work per cycle is required each cycle to run this refrigerator?
(b)What is the coefficient of performance of this refrigerator?
(Short Answer)
4.9/5  (39)
(39)
An ideal gas undergoes the process a?b?c?a shown in the pV diagram. The heat gained by the gas in process a?b is 546 J, while in process the gas loses 62.0 J of heat. In process a?b the gas performs of work, while in process c?a 223 J of work is done on the gas. How much heat is gained by the gas in process c?a? 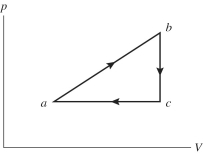
(Multiple Choice)
4.8/5  (40)
(40)
A person running in place on an exercise machine for 10 min uses up 17 kcal (food calories). Another person exercises by repeatedly lifting two 2.5-kg weights a distance of 50 cm. How many repetitions of this exercise are equivalent to 10 minutes of running in place? Assume that the person uses negligible energy in letting down the weights after each lift. (1 cal = 4.186 J)
(Multiple Choice)
4.8/5  (41)
(41)
The "hot shot" heat engine operating between 40°C and 380°C has an efficiency that is 60% of that of an ideal Carnot engine operating between the same temperatures. If the "hot shot" engine absorbs heat at a rate of 60 kW, at what rate does it exhaust heat?
(Multiple Choice)
4.9/5  (33)
(33)
An external heat source supplies heat to a system at a rate of 187 W as the system does work at a rate of 131 W. At what rate is the internal (thermal)energy of the system changing?
(Multiple Choice)
4.9/5  (39)
(39)
What is absolute zero on the (a)Celsius scale and (b)on the Fahrenheit scale?
(Short Answer)
4.7/5  (41)
(41)
Nitrogen boils at -196°C. What is the corresponding temperature in the Fahrenheit scale?
(Multiple Choice)
4.7/5  (32)
(32)
In an adiabatic compression, 200 J of work is done on a gas. What is the change in internal (thermal)energy of the gas during this compression?
(Multiple Choice)
4.7/5  (37)
(37)
A Carnot engine operates between two reservoirs with unknown temperatures. If the Carnot engine operates at efficiency, what is the ratio of the absolute temperatures of the reservoirs, Tc/Th?
(Multiple Choice)
4.8/5  (38)
(38)
An ideal Carnot heat engine operates between reservoirs at 1740 K and In each cycle, 260 J of heat energy is rejected to the low temperature reservoir. In each cycle, how much mechanical work W is performed by the engine?
(Short Answer)
4.7/5  (30)
(30)
The ocean thermal energy conversion project uses the surface water near tropical islands with a temperature of 20°C as the hot temperature reservoir, and the water at some depth, with a temperature of 5.0°C, as the cold temperature reservoir for a heat engine. What is the maximum possible efficiency of an engine running between those two temperatures?
(Multiple Choice)
4.8/5  (43)
(43)
A heat engine having the maximum possible efficiency has an efficiency of 35.0% when operating between two heat reservoirs. If the temperature of the hot reservoir is 700 K, what is the temperature of the cold reservoir?
(Multiple Choice)
4.8/5  (48)
(48)
Showing 41 - 60 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)