Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A 5.3 L flask of ideal neon gas (which is monatomic)is at a pressure of 6.0 atm and a temperature of The atomic mass of neon is 20.2 g/mol. What is the mass of the neon gas in the flask. (R = 8.31 J/mol ? K, 1 atm = 101 kPa, NA = 6.022 × 1023 molecules/mol)
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
A
A 600-g piece of iron at 100°C is dropped into a calorimeter of negligible heat capacity containing 100 g of ice at 0°C and 120 g of water, also at 0°C. What is the final temperature of the system? The specific heat of iron is 448 J/kg ∙ K, the latent heat of fusion of water is 33.5 × 104 J/kg, and the specific heat of water is 4186 J/kg ∙ K.
Free
(Essay)
4.9/5  (42)
(42)
Correct Answer:
0°C (only some of the ice will have melted)
If the temperature of an ideal gas is increased from 20°C to 40°C, by what percent does the speed of the molecules increase?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
It is necessary to determine the specific heat of an unknown object. The mass of the object is It is determined experimentally that it takes to raise the temperature What is the specific heat of the object?
(Multiple Choice)
4.8/5  (43)
(43)
If you add 700 kJ of heat to 700 g of water originally at 70.0°C, how much water is left in the container? The latent heat of vaporization of water is 22.6 × J/kg, and its specific heat capacity is 4186 J/kg ? K.
(Multiple Choice)
4.8/5  (35)
(35)
A quantity of mercury occupies 400.0 cm3 at 0°C. What volume will it occupy when heated to 50°C? Mercury has a volume expansion coefficient of 180 × 10-6 K-1.
(Multiple Choice)
4.7/5  (30)
(30)
The coefficient of linear expansion of steel is 12 × 10-6 K-1. What is the change in length of a 25-m steel bridge span when it undergoes a temperature change of 40 K from winter to summer?
(Multiple Choice)
4.7/5  (39)
(39)
An oxygen molecule, O2, falls in a vacuum. From what height must it fall so that its translational kinetic energy at the bottom of its fall equals the average translational kinetic energy of an oxygen molecule at 920 K? The mass of one O2 molecule is 5.312 × 10-26 kg, and the Boltzmann constant is 1.38 × 10-23 J/K. Neglect air resistance and assume that g remains constant at 9.8 m/s2 throughout the fall of the molecule.
(Multiple Choice)
4.7/5  (43)
(43)
The figure shows a pV diagram for an ideal gas that is carried around a cyclic process. How much work is done in one cycle if p0 = and L? (1.00 atm = 101 kPa) 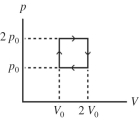
(Multiple Choice)
4.8/5  (36)
(36)
The rms speed of a certain sample of carbon dioxide molecules, with a molecular weight of 44.0 g/mole, is 396 m/s. What is the rms speed of water vapor molecules, with a molecular weight of 18.0 g/mol, at the same temperature as the carbon dioxide?
(Multiple Choice)
4.9/5  (33)
(33)
Heat is added to a 3.0 kg piece of ice at a rate of How long will it take for the ice at 0.0° C to melt? For water LF = 334,000 J/kg and LV = 2.246 × 106 J/kg.
(Multiple Choice)
4.9/5  (33)
(33)
The temperature of an ideal gas in a sealed rigid 0.60- container is reduced from 460 K to The final pressure of the gas is The molar heat capacity at constant volume of the gas is 28.0 J/mol ? K. How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ? K)
(Multiple Choice)
4.8/5  (33)
(33)
Two metal rods are to be used to conduct heat from a region at 100°C to a region at 0°C as shown in the figure. The rods can be placed in parallel, as shown on the left, or in series, as on the right. When steady state flow is established, the heat conducted in the series arrangement is 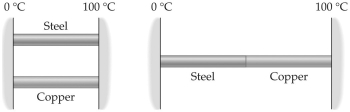
(Multiple Choice)
4.8/5  (37)
(37)
A container with rigid walls is filled with 4.00 mol of air at 17°C with CV = 2.5R. What is the final temperature of the air if its internal energy is increased by 28 kJ? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (37)
(37)
Object 1 has three times the specific heat capacity and four times the mass of Object 2. The two objects are heated from the same initial temperature, T0, to the same final temperature Tf. During this process, if Object 1 absorbs heat Q, the amount of heat absorbed by Object 2 will be
(Multiple Choice)
4.8/5  (47)
(47)
Your lungs hold 4.2 L of air at a temperature of 27°C and a pressure of 101.3 kPa. How many moles of air do your lungs hold? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (41)
(41)
The process in which heat flows by the mass movement of molecules from one place to another is known as
(Multiple Choice)
4.9/5  (40)
(40)
A piece of iron of mass 0.12 kg is taken from an oven where its temperature is 336°C and quickly placed in an insulated copper can that contains 0.20 kg of water. The copper can has mass 0.50 kg, and it and the water in it are originally at a temperature of 20°C. Calculate the final temperature of the system, assuming no heat is lost to the surroundings. Use the following specific heats: 4190J/kg ? C° (water), 470 J/kg ? C° (iron), and 390 J/kg ? C° (copper).
(Short Answer)
4.9/5  (30)
(30)
How many grams of ice at -17°C must be added to 741 grams of water that is initially at a temperature of to produce water at a final temperature of Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg ? C° and of ice is 2000 J/kg ? C°. For water the normal melting point is 0°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.256 × 106 J/kg.
(Short Answer)
4.8/5  (32)
(32)
Showing 1 - 20 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)