Exam 9: Molecular Geometry: Shape Determines Function
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Use the 1s orbital of hydrogen to form atomic orbitals for H2 and draw the molecular orbital energy-level diagram. Label the energy levels with the MO symbols. Which of the following species of hydrogen would you predict to be stable based on your energy-level diagram? Explain.
H22+, H2+, H2, H2-, H22-
Free
(Essay)
4.9/5  (35)
(35)
Correct Answer:
*________
________
Bond orders are 0 for H22+; 0.5 for H2+; 1 for H2; 0.5 for H2-; 0 for H22-. Expect those species with bond orders greater than 0 to be stable.
Identify the local molecular geometry and hybridization at atoms a and b in Ritalin, shown below, which is a drug used to treat attention deficit hyperactivity disorder. In the line drawing, the hydrogen atoms bonded to carbon are not shown explicitly. 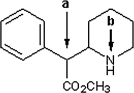
Free
(Essay)
4.8/5  (32)
(32)
Correct Answer:
Both atoms a and b are sp3 hybridized. Atom a has a tetrahedral local molecular geometry, and atom b has a local molecular geometry that forms a trigonal pyramid.
Why does the seesaw molecular geometry (ss) have a lower energy than the trigonal pyramidal molecular geometry (tp) in an AB4 molecule with a trigonal bipyramidal electron-pair geometry?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
A
Which of the following molecules has a central atom that is sp3 hybridized?
(Multiple Choice)
4.9/5  (32)
(32)
Identify the hybridization of atomic orbitals for atoms a, b, and c in the structure below of acetaminophen, which is the active ingredient in the analgesic Tylenol. 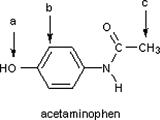
(Short Answer)
4.8/5  (33)
(33)
Which of the following molecules or ions has a central atom with the same hybridization as PCl5?
(Multiple Choice)
4.8/5  (36)
(36)
Boron nitride is being investigated in frontier research directed at producing novel electronic devices. If you used the following energy-level diagram for the molecular orbitals of boron nitride, BN, what would you predict? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 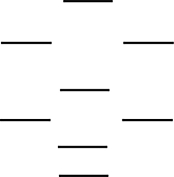 I. Boron nitride is diamagnetic.
II) Boron nitride has a bond order of 2.
III) Boron nitride is paramagnetic.
IV) The bond in BN- is stronger than the bond in BN.
I. Boron nitride is diamagnetic.
II) Boron nitride has a bond order of 2.
III) Boron nitride is paramagnetic.
IV) The bond in BN- is stronger than the bond in BN.
(Multiple Choice)
4.9/5  (31)
(31)
Benzene (C6H6) is a cyclic, nonpolar molecule. By removing one of the hydrogens and replacing it with another atom or group, this substituted benzene becomes polar. Which of the following substituted benzenes is the most polar?
(Multiple Choice)
4.9/5  (40)
(40)
Both diazene (N2H2) and hydrazine (N2H4) have two nitrogen atoms bonded together. In a valence bond picture of the N-N bonds, ________ hybrid orbitals overlap for diazene and ________ hybrid orbitals overlap for hydrazine.
(Multiple Choice)
4.7/5  (36)
(36)
Draw a structure showing the geometry of the molecule in the following group that is nonpolar:
HF; NH3; BF3; CHCl3; CO.
(Essay)
4.9/5  (36)
(36)
Which statement regarding a pi bond between two carbon atoms is correct?
(Multiple Choice)
4.7/5  (35)
(35)
Both cyclohexane (C6H12) and benzene (C6H6) have the carbon atoms forming a six-member ring. In a valence bond picture of the C-C bonds, ________ hybrid orbitals would overlap for cyclohexane, and ________ hybrid orbitals would overlap for benzene.
(Multiple Choice)
4.8/5  (38)
(38)
Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below. Explain why you think this molecule is planar or nonplanar. 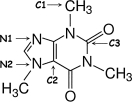
(Essay)
4.9/5  (32)
(32)
Showing 1 - 20 of 136
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)