Exam 10: Intermolecular Forces: The Uniqueness of Water
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which of the following nonpolar molecules will have the highest boiling point?
Free
(Multiple Choice)
5.0/5  (37)
(37)
Correct Answer:
C
For each of the following pairs of compounds, identify the one that is more likely to be soluble in water. Explain the rationale for your choice.
(A) CCl4 or CHCl3
(B) CH3OH or C5H11OH
(C) NaF or MgO
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
(A) CHCl3, because it has a dipole moment to interact more strongly with water. CCl4 does not have a dipole moment.
(B) CH3OH, because it has a dipole moment, can hydrogen-bond with water, and has a small hydrocarbon part. C5H11OH has a large hydrocarbon part, so it disrupts the hydrogen bonding in water, which is unfavorable.
(C) NaF, because the ions only have single charges so the attractions are more easily overcome than in MgO, where ionic charges are 2+ and 2-.
Boiling points increase in the order HCl < HBr < HI because ________ contribute to the intermolecular interactions.
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
Which of the following compounds do you expect to be most soluble in water?
(Multiple Choice)
4.8/5  (35)
(35)
Of all the noble gases, ________ has the strongest intermolecular force and hence the highest boiling point.
(Multiple Choice)
4.8/5  (41)
(41)
The resistance of a liquid to an increase in its surface area is ________
(Multiple Choice)
4.9/5  (40)
(40)
Which is the dominant interaction between carbon dioxide molecules?
(Multiple Choice)
4.9/5  (36)
(36)
Dipole-dipole interactions typically are not as strong as ion-dipole interactions because ________
(Multiple Choice)
4.8/5  (37)
(37)
Explain why the boiling point of nitrogen (N2, 77 K) is much lower than that of carbon monoxide (CO, 84 K), even though both have the same number of electrons and protons and nearly the same mass.
(Essay)
5.0/5  (32)
(32)
Predict which of the following ionic compounds has the highest melting point.
(Multiple Choice)
4.7/5  (41)
(41)
Which is the dominant interaction that explains the high melting point of table salt, NaCl?
(Multiple Choice)
4.8/5  (34)
(34)
What does the line indicated by the arrow in the following phase diagram represent? 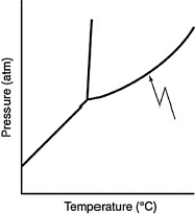
(Multiple Choice)
4.9/5  (28)
(28)
At the point marked with a dot on the phase diagram, the solid will ________ 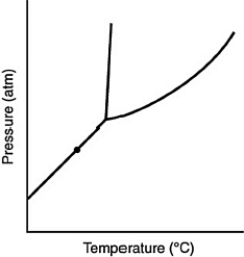
(Multiple Choice)
5.0/5  (31)
(31)
Portable lanterns and stoves used for camping often use a mixture of hydrocarbons for fuel. One such hydrocarbon is an alkane called n-pentane. These lanterns and stoves are often difficult to light on a cold day because the fuel has a low vapor pressure at low temperatures. Determine the vapor pressure of n-pentane on a night when the temperature is 0.0°C. The enthalpy of vaporization of n-pentane is 27.6 kJ/mol, and its boiling point is 36.0°C.
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following statements does not correctly identify a factor that affects the boiling point of a pure substance?
I. vapor pressure of the liquid
II. strength of intermolecular forces
III. enthalpy of vaporization
IV. surface area of the liquid
(Multiple Choice)
4.8/5  (45)
(45)
Showing 1 - 20 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)