Exam 5: Changes of State and the Gas Laws
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
The atmospheric pressure in Denver is 0.85 atm. What is the atmospheric pressure in torr?
(Multiple Choice)
4.9/5  (34)
(34)
An oxygen tank has a pressure of 12 atm at 295 K. The maximum pressure that the tank can hold is 25 atm. What is the maximum temperature at which the tank can be stored?
(Multiple Choice)
4.8/5  (40)
(40)
You drive your car from Salt Lake City up into the mountains to go skiing. It is a nice day in Salt Lake, with a temperature of 16C and an atmospheric pressure of 0.85 atm. Up in the mountains, it is freezing at 1.0C and an atmospheric pressure of 0.70 atm. If the volume of air in your tires is 12 L when you leave the city, what is it in the mountains?
(Multiple Choice)
4.9/5  (38)
(38)
Henry's law is P = kC. Which statement best describes the meaning of this law?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following are ways to increase the pressure of a gas?
(Multiple Choice)
4.7/5  (33)
(33)
What change of phase is represented by B on the heating curve? 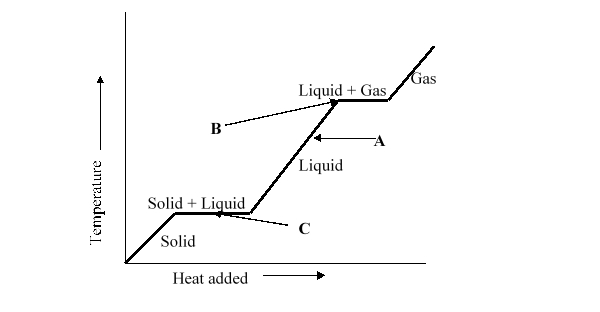
(Multiple Choice)
4.9/5  (41)
(41)
In which of the following statements is the gas variable correctly described?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following changes is not correctly labeled as a chemical reaction or a change of state?
(Multiple Choice)
4.8/5  (35)
(35)
How does the pressure of a gas change when it is compressed?
(Multiple Choice)
4.9/5  (42)
(42)
You are riding your bike and run over a thorn, puncturing your tire. The air in the tire is released, resulting in a flat. What is the relationship between the volume (Vi) and moles of air (ni) in the inflated tire and the volume (Vf) and moles of air in the flat tire (nf)?
(Multiple Choice)
4.8/5  (31)
(31)
According to Avogadro's Law, What is the meaning of this law?
(Multiple Choice)
5.0/5  (38)
(38)
Which process requires more energy per gram: melting ice or boiling water?
(Multiple Choice)
4.9/5  (34)
(34)
A narrow tube on a road bike should be inflated to about 100 psi. What is this pressure in atmospheres?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following statements best describes vapor pressure?
(Multiple Choice)
4.9/5  (37)
(37)
Balloon A is placed into a container that has a higher pressure than atmospheric pressure. Which balloon would you predict to be the new size of balloon A? 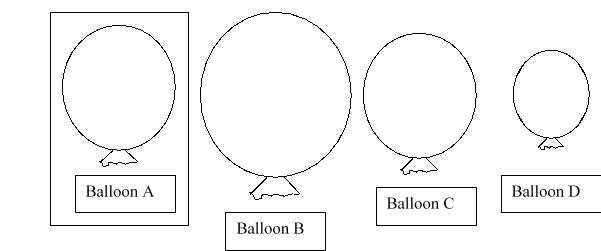
(Multiple Choice)
4.9/5  (35)
(35)
What occurs over the course of this physical change illustrated below? 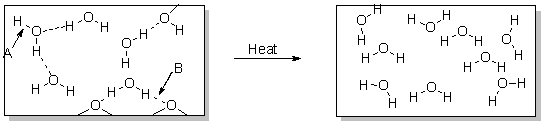
(Multiple Choice)
4.8/5  (38)
(38)
Which statement best describes how heat is involved in the change in the diagram below? 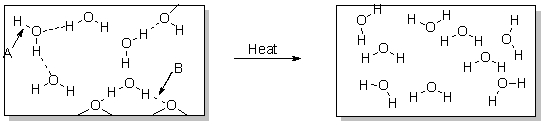
(Multiple Choice)
4.8/5  (43)
(43)
Showing 21 - 40 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)