Exam 9: Acids and Bases
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
Which of the following is a buffer system?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
A
Which of the following statements best describe a neutralization reaction?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
A
Generally, strong bases are hydroxide salts of
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
A weak acid is also a _________ because it produces a low concentration of ions in solution.
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following solutions has the highest concentration of [OH]?
(Multiple Choice)
4.9/5  (38)
(38)
The following is the reaction of benzoic acid in water. Which of the following choices is a conjugate acid-base pair in this reaction? 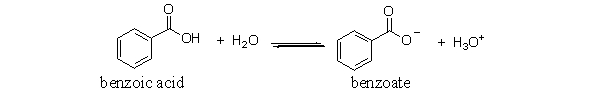
(Multiple Choice)
4.9/5  (40)
(40)
In the acid-base reaction between ammonia and water, which of the following statements best describes the concentration of ammonia and ammonium at equilibrium? 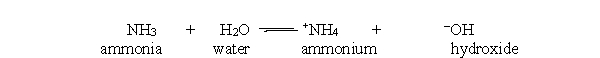
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following equations best describes what will happen to formic acid when it reacts with water?
(Multiple Choice)
4.8/5  (38)
(38)
A buffer solution contains H2CO3 and HCO3, resulting in the equilibrium shown by the reaction below. According to this reaction and LeChatelier's principle, what happens when acid (H3O+) is added to this buffer solution? 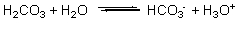
(Multiple Choice)
4.8/5  (50)
(50)
What is the pH of a solution with a [H3O+] of 1.0 105 M?
(Multiple Choice)
4.9/5  (36)
(36)
Which substance is acting as the acid in the reverse reaction of the acid-base reaction below? 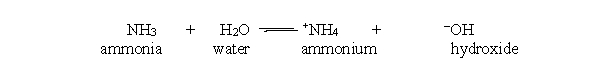
(Multiple Choice)
5.0/5  (29)
(29)
According to the Arrhenius definition, which of the following compounds is a base? 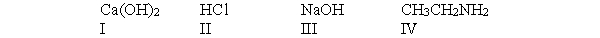
(Multiple Choice)
4.8/5  (35)
(35)
Acetic acid (CH3COOH) has a pKa of 4.74, while ethanol (CH3CH2OH) has a pKa of about 16. Which molecule is more acidic?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the statements describes the following reaction? Mg(OH)2 → Mg2+ + 2 HO
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 112
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)