Exam 5: Changes of State and the Gas Laws
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
What is the strongest intermolecular that exists between molecules of acetone in the liquid or solid state? 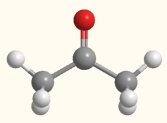
(Multiple Choice)
4.9/5  (34)
(34)
Does isopropyl alcohol have a higher or lower boiling point than acetone? 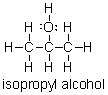
(Multiple Choice)
4.8/5  (32)
(32)
A sample of gaseous neon has a volume of 68.2 L at STP. How many moles of neon are in the sample?
(Multiple Choice)
5.0/5  (38)
(38)
What is the molecular geometry of the carbon indicated by the arrow in this molecule of acetone? 
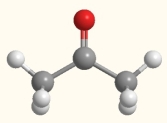
(Multiple Choice)
4.8/5  (44)
(44)
Which has a higher partial pressure of oxygen: inhaled air or exhaled air?
(Multiple Choice)
4.7/5  (36)
(36)
A liquid has temperature A as shown on the heating curve. What will happen to the temperature of the liquid if heat is added to it? 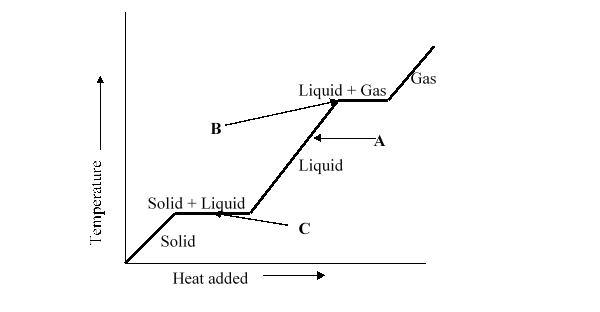
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following is NOT a common use of a hyperbaric oxygen therapy (HBOT) chamber?
(Multiple Choice)
4.7/5  (38)
(38)
You boil a pot of water (H2O) to make spaghetti and, when the water reaches its boiling point, you notice that it bubbles. What are these bubbles composed of?
(Multiple Choice)
4.7/5  (45)
(45)
Which of the following instruments is used to measure atmospheric pressure?
(Multiple Choice)
4.7/5  (36)
(36)
On a cool morning (12C), a balloon is filled with 1.5 L of helium. By mid-afternoon, the temperature has soared to 32C. What is the new volume of the balloon?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following properties is not a property of gases?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following statements about the relationship between pressure and temperature is true?
(Multiple Choice)
4.9/5  (33)
(33)
Which statement best describes why steam burns are more severe than boiling water burns?
(Multiple Choice)
4.7/5  (35)
(35)
Which statement best describes how heat energy is involved in changing water into steam?
(Multiple Choice)
4.8/5  (34)
(34)
What is the electron geometry of the carbon indicated by the arrow in this molecule of acetone? 
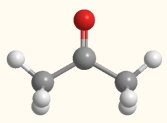
(Multiple Choice)
4.9/5  (36)
(36)
The temperature of a gas at 1.00 atm and 8.00C is increased to 20.0C, resulting in a change of pressure. Which equation would you use to calculate the new pressure?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following types of molecules would you expect to have the highest vapor pressure at a given temperature?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following statements best describes how hyperbaric oxygen therapy is used to treat carbon monoxide poisoning?
(Multiple Choice)
4.9/5  (44)
(44)
One of the symptoms of the bends is joint pain. Why does joint pain occur with the bends?
(Multiple Choice)
4.8/5  (46)
(46)
Showing 61 - 80 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)