Exam 5: Changes of State and the Gas Laws
Exam 1: Measuring Matter and Energy99 Questions
Exam 2: Atomic Structure and Nuclear Radiation135 Questions
Exam 3: Compounds and Molecules164 Questions
Exam 4: Chemical Quantities and Chemical Reactions135 Questions
Exam 5: Changes of State and the Gas Laws85 Questions
Exam 6: Organic Chemistry: Hydrocarbons95 Questions
Exam 7: Organic Chemistry and Biomolecules101 Questions
Exam 8: Solutions, Colloids and Membranes93 Questions
Exam 9: Acids and Bases112 Questions
Exam 10: The Reactions of Organic Functional Groups in Biochemistry87 Questions
Exam 11: Carbohydrates: Structure and Function78 Questions
Exam 12: Lipids: Structure and Function108 Questions
Exam 13: Proteins: Structure and Function122 Questions
Exam 14: Nucleotides and Nucleic Acids107 Questions
Exam 15: Energy and Metabolism117 Questions
Select questions type
Which bond in acetone, shown below, is correctly labeled with a dipole arrow? 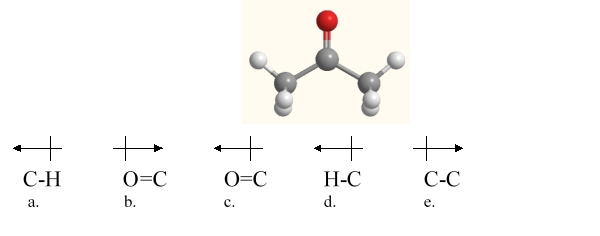
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
C
What happens to the speed of molecules when the molecules are frozen?
Free
(Multiple Choice)
4.8/5  (46)
(46)
Correct Answer:
C
Which of the following statements best describes the process that occurs when air is inhaled into the lungs?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
Three boxes, each containing molecules in the gas phase, are illustrated below. Which box would you expect to have the highest pressure? 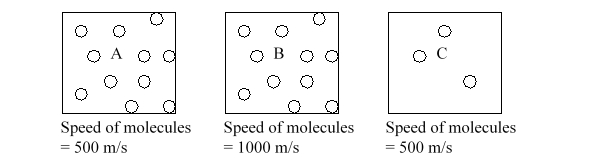
(Multiple Choice)
4.8/5  (37)
(37)
Imagine that you have a beaker of gas molecules. A small volume of the gas in the beaker is enlarged so you can see the gas particles. If all of the gas in the beaker A is transferred to a new beaker half the size of the original beaker, while maintaining the same temperature, which magnified view best represents what the gas would look like? 
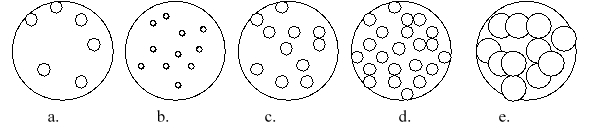
(Multiple Choice)
4.9/5  (30)
(30)
Hyperbaric oxygen therapy is the use of ________ to treat a variety of medical conditions.
(Multiple Choice)
4.7/5  (39)
(39)
It is important to check tire pressure periodically, especially when the outside temperature drops. You can see why this is so by calculating the tire volume after a large drop in temperature as follows: A well-inflated tire holds about 11 L of air at 298 K (25C). If the temperature drops to 273 K (0C), what is the new volume of the air in the tire?
(Multiple Choice)
4.7/5  (39)
(39)
Which statement best describes why it is important to know the vapor pressure of a material before working with it?
(Multiple Choice)
4.7/5  (29)
(29)
A nitrogen bubble with a volume of 0.0050 mL forms in the joint of a scuba diver as she ascends rapidly from a pressure of 4.1 atm to the surface, with a pressure of 1.0 atm. What is the volume of the bubble at the surface?
(Multiple Choice)
4.8/5  (41)
(41)
Henry's constant for halothane, an anesthetic, is higher than that of ether. Which anesthetic would you expect to be faster acting?
(Multiple Choice)
4.8/5  (37)
(37)
What happens when a large volume of gas is compressed to a smaller volume??
(Multiple Choice)
4.9/5  (37)
(37)
To accomplish the change of phase shown in the diagram below, you could 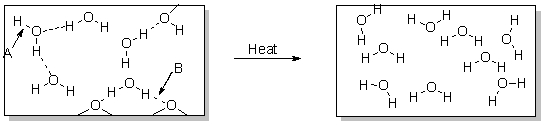
(Multiple Choice)
4.8/5  (38)
(38)
The total pressure in a mixture of gases is equal to the partial pressure(s) of
(Multiple Choice)
4.8/5  (39)
(39)
This diagram shows balloons of various sizes filled with helium gas. Balloon A is at STP. If you heat balloon A, which balloon would you predict would best represent the new size of balloon A? 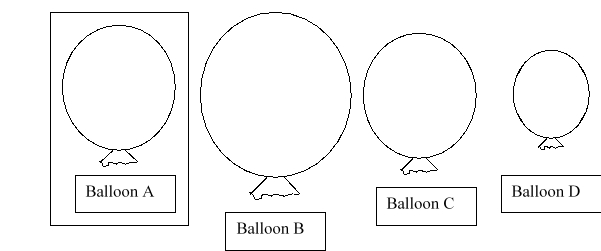
(Multiple Choice)
4.9/5  (41)
(41)
Acetone, shown here, is a common solvent and component of fingernail polish remover. Which of the following Lewis structures is acetone? 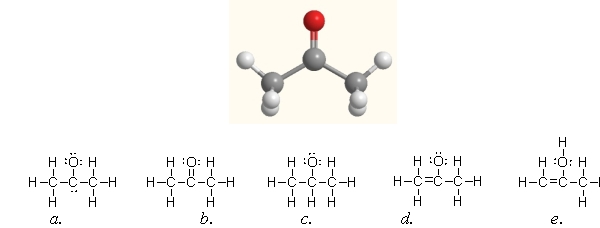
(Multiple Choice)
4.7/5  (48)
(48)
Showing 1 - 20 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)