Exam 24: Nuclear Reactions and Their Applications
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
The radiochemist, Will I. Glow, studied thorium-232 and found that 2.82 *10¯7 moles emitted 8.42 *106 particles in one year. What is the decay constant for thorium-232?
(Multiple Choice)
4.8/5  (33)
(33)
Write a complete, balanced equation to represent the beta decay of thallium-207.
(Short Answer)
4.9/5  (37)
(37)
Which one of the following is an incorrect representation of the indicated particle or nucleus?
(Multiple Choice)
4.8/5  (39)
(39)
Select the nuclide that completes the following nuclear reaction: 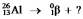
(Multiple Choice)
4.8/5  (36)
(36)
Write a complete, balanced equation to represent the formation of manganese-55 by the beta decay of another nuclide.
(Short Answer)
4.8/5  (35)
(35)
Positron decay and electron capture have the same net effect on the Z and N values of a nucleus.
(True/False)
4.9/5  (43)
(43)
Select the nuclide that completes the following nuclear reaction: 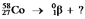
(Multiple Choice)
4.8/5  (36)
(36)
Which one of the following is a subatomic particle closely related to the positron?
(Multiple Choice)
4.9/5  (36)
(36)
Fill in missing sub- and superscripts for all particles to complete the following equation for beta decay. 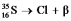
(Short Answer)
4.9/5  (37)
(37)
Identify the missing species in the following nuclear transmutation: 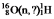
(Multiple Choice)
4.7/5  (43)
(43)
The isotopes 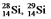 and
and 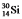 are all stable, while
are all stable, while 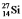 is radioactive. The mode of decay for
is radioactive. The mode of decay for 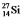 is most likely to be
is most likely to be
(Multiple Choice)
5.0/5  (39)
(39)
The (negative) binding energy per nucleon reaches a maximum for the isotope 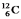 .
.
(True/False)
4.8/5  (36)
(36)
A 7.85 *10¯5 mol sample of copper-61 emits 1.47 *1019 positrons in 90.0 minutes. What is the decay constant for copper-61?
(Multiple Choice)
4.9/5  (32)
(32)
Select the nuclide that completes the following nuclear reaction: 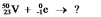
(Multiple Choice)
4.8/5  (36)
(36)
No alpha decay is observed for isotopes of elements with Z < 83.
(True/False)
4.8/5  (39)
(39)
Showing 41 - 60 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)