Exam 24: Nuclear Reactions and Their Applications
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
A certain isotope has a specific activity of 7.29 * 10¯4 Ci/g. How many particles will a 75.0 mg sample emit in one hour?
(Multiple Choice)
4.7/5  (33)
(33)
Select the nuclide that completes the following nuclear reaction: 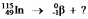
(Multiple Choice)
4.9/5  (33)
(33)
What is the specific activity (in Ci/g) of an isotope if 3.56 mg emits 4.26* 108 particles per second?
(Multiple Choice)
5.0/5  (27)
(27)
The s-process involves a slow succession of neutron absorption and beta decay processes during the normal life of a star.
(True/False)
4.7/5  (30)
(30)
Fill in missing sub- and superscripts for all particles to complete the following equation for alpha decay. 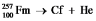
(Short Answer)
4.9/5  (40)
(40)
A N-14 nucleus is hit by a particle, forming a C-14 nucleus and a proton as the only products. Identify the type of particle which struck the N-14 nucleus.
(Multiple Choice)
4.9/5  (38)
(38)
In living organisms, C-14 atoms disintegrate at a rate of 15.3 atoms per minute per gram of carbon. A charcoal sample from an archaeological site has a C-14 disintegration rate of 9.16 atoms per minute per gram of carbon. Estimate the age of this sample. The half-life of C-14 is 5730 years.
(Multiple Choice)
4.7/5  (30)
(30)
Sodium-21 will emit positrons each having an energy of 4.0 *10¯13 J. What is this energy in MeV?
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the following elements is formed largely in supernova explosions?
(Multiple Choice)
4.8/5  (43)
(43)
Which one of the following equations correctly represents positron decay of 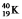 ?
?
(Multiple Choice)
4.8/5  (41)
(41)
Write a complete, balanced equation to represent the alpha decay of radon-210.
(Short Answer)
4.9/5  (38)
(38)
A patient's thyroid gland is to be exposed to an average of 5.5 µCi for 16 days as an ingested sample of iodine-131 decays. If the energy of the radiation is 9.7 * 10¯14 J and the mass of the thyroid is 32.0 g, what is the dose received by the patient?
(Multiple Choice)
4.8/5  (35)
(35)
An isotope with Z > 83, which lies close to the band of stability, will generally decay through
(Multiple Choice)
4.9/5  (43)
(43)
Which one of the following equations correctly represents electron capture by the 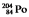 nucleus?
nucleus?
(Multiple Choice)
4.7/5  (37)
(37)
Select the nuclide that completes the following nuclear reaction: 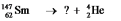
(Multiple Choice)
4.9/5  (41)
(41)
Iodine-131, t1/2= 8.0 days, is used in diagnosis and treatment of thyroid gland diseases. If a laboratory sample of iodine-131 initially emits 9.95 *1018 particles per day, how long will it take for the activity to drop to 6.22 * 1017 particles per day?
(Multiple Choice)
4.8/5  (41)
(41)
What is the mechanism by which control rods slow down the fission rate in a nuclear reactor?
(Essay)
4.8/5  (38)
(38)
Showing 61 - 80 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)