Exam 23: Substitution Reactions of Carbonyl Compounds at the Alpha-Carbon
Exam 1: Structure and Bonding69 Questions
Exam 2: Acids and Bases52 Questions
Exam 3: Introduction to Organic Molecules and Functional Groups45 Questions
Exam 4: Alkanes57 Questions
Exam 5: Stereochemistry59 Questions
Exam 6: Understanding Organic Reactions45 Questions
Exam 7: Alkyl Halides and Nucleophilic Substitution61 Questions
Exam 8: Alkyl Halides and Elimination Reactions43 Questions
Exam 9: Alcohols, Ethers, and Related Compounds49 Questions
Exam 10: Alkenes43 Questions
Exam 11: Alkynes42 Questions
Exam 12: Oxidation and Reduction39 Questions
Exam 13: Mass Spectrometry and Infrared Spectroscopy35 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy29 Questions
Exam 15: Radical Reactions42 Questions
Exam 16: Conjugation, Resonance, and Dienes43 Questions
Exam 17: Benzene and Aromatic Compounds31 Questions
Exam 18: Reactions of Aromatic Compounds54 Questions
Exam 19: Carboxylic Acids and the Acidity of the O-H Bond36 Questions
Exam 20: Introduction to Carbonyl Chemistry;29 Questions
Exam 21: Aldehydes and Ketones Nucleophilic Addition42 Questions
Exam 22: Carboxylic Acids and Derivatives46 Questions
Exam 23: Substitution Reactions of Carbonyl Compounds at the Alpha-Carbon40 Questions
Exam 24: Carbonyl Condensation Reactions45 Questions
Exam 25: Amines53 Questions
Exam 26: Carbon-Carbon Bond Forming Reactions in Organic Synthesis37 Questions
Exam 27: Pericyclic Reactions47 Questions
Exam 28: Carbohydrates38 Questions
Exam 29: Amino Acids and Proteins35 Questions
Exam 30: Synthetic Polymers36 Questions
Exam 31: Lipids39 Questions
Select questions type
Why is the enolate of acetone less basic than the allyl anion derived from propene?
(Multiple Choice)
4.8/5  (37)
(37)
Why is it difficult to stop the halogenation of ketones under basic conditions at the mono-halogenated stage?
(Multiple Choice)
4.8/5  (52)
(52)
Vitamin C is a stable enediol.Which is the structure of a possible keto form in equilibrium with the enediol form?
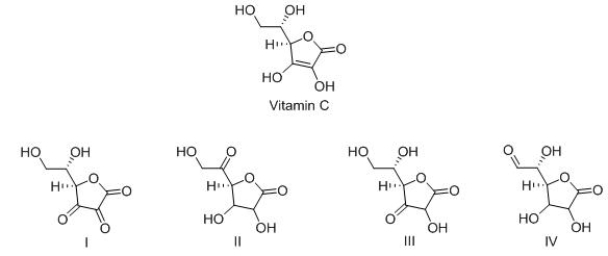
(Multiple Choice)
4.8/5  (36)
(36)
Starting with cyclohexanone, how could you prepare the diketone below?
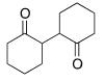
(Multiple Choice)
4.8/5  (33)
(33)
Will acetophenone be completely deprotonated by lithium diisopropylamide (LDA)?
(Multiple Choice)
4.8/5  (34)
(34)
The reaction below is a direct enolate alkylation.It has been found that this reaction only works well with unhindered methyl and 1° alkyl halides.Pick the statement that best explains this observation. 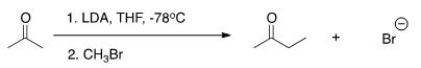
(Multiple Choice)
4.9/5  (44)
(44)
The malonic ester synthesis can be adapted to synthesize a-amino acids by using diethyl acetamidomalonate as the starting material.Select the structure of the amino acid produced by the following synthesis. 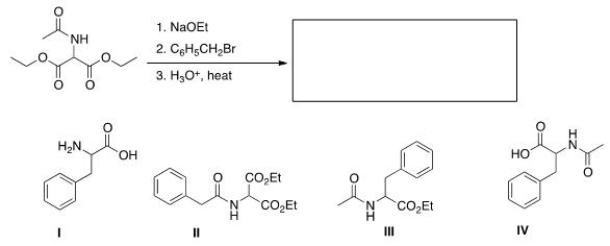
(Multiple Choice)
4.8/5  (41)
(41)
Will acetone be completely deprotonated by potassium tert-butoxide?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following are enol forms of ethyl acetoacetate drawn below?
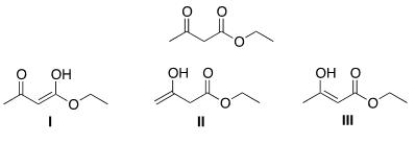
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following compounds is an enol of compound X drawn below?
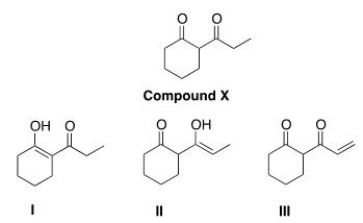
(Multiple Choice)
4.9/5  (40)
(40)
Select the appropriate sequence of reactions to accomplish the following synthesis. 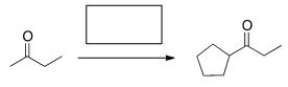
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following ketones will give a positive iodoform test?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 21 - 40 of 40
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)