Exam 10: The Liquid and Solid States
Exam 1: Matter and Energy110 Questions
Exam 2: Atoms, Ions, and the Periodic Table135 Questions
Exam 3: Chemical Compounds112 Questions
Exam 4: Chemical Composition111 Questions
Exam 5: Chemical Reactions and Equations108 Questions
Exam 6: Quantities in Chemical Reactions120 Questions
Exam 7: Electron Structure of the Atom116 Questions
Exam 8: Chemical Bonding114 Questions
Exam 9: The Gaseous State116 Questions
Exam 10: The Liquid and Solid States107 Questions
Exam 11: Solutions110 Questions
Exam 12: Reaction Rates and Chemical Equilibrium52 Questions
Exam 13: Acids and Bases128 Questions
Exam 14: Oxidation-Reduction Reactions117 Questions
Exam 15: Nuclear Chemistry88 Questions
Exam 16: Organic Chemistry119 Questions
Exam 17: Biochemistry95 Questions
Select questions type
Calculate the amount of heat energy, in units of kilojoules, required to melt 75.0 g of ice at 0.0 C.(molar heat of fusion of ice = 6.01 x 103 J/mol)
(Multiple Choice)
4.9/5  (39)
(39)
Detergents are used to increase the surface tension of water, so that it will stay in the shape of a droplet.
(True/False)
4.8/5  (35)
(35)
The diagram represents the physical state of a substance.Where does this physical state occur on the heating curve? 
(Multiple Choice)
4.8/5  (26)
(26)
The diagram represents the physical state of a substance.Where does this physical state occur on the cooling curve? 
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
(Multiple Choice)
4.9/5  (39)
(39)
The diagram represents the physical state of a substance.Where does this physical state occur on the heating curve? 
(Multiple Choice)
4.8/5  (29)
(29)
Which choice correctly lists the intermolecular forces present in CH3Br?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following has the strongest London dispersion forces?
(Multiple Choice)
4.7/5  (30)
(30)
A solid substance has a very low melting point, is soft, and does not conduct heat or electricity.This substance is probably a(n)________ solid.
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following statements regarding intermolecular forces is incorrect?
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the amount of heat energy required to evaporate 27.5 g of water at 100.0 C.(molar heat of vaporization of liquid water = 4.07 x 104 J/mol)
(Multiple Choice)
4.9/5  (30)
(30)
Of the two molecules shown in the figure, one would expect that CO2 has a higher boiling point than NO2 because CO2 is linear and nonpolar. 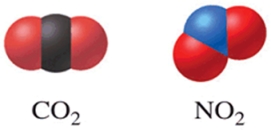
(True/False)
4.9/5  (29)
(29)
Which of the following statements regarding the liquid state is incorrect?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following substances is most likely to be a gas at room temperature?
(Multiple Choice)
4.7/5  (29)
(29)
From the vapor pressure curve of propane, it can be seen that the normal boiling point of propane is about _________. 
(Multiple Choice)
4.8/5  (33)
(33)
What phase change is occurring in the figure, and is it endothermic or exothermic? 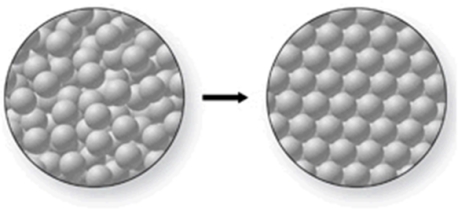
(Multiple Choice)
4.9/5  (41)
(41)
Which choice correctly lists the intermolecular forces present in CH4?
(Multiple Choice)
4.8/5  (37)
(37)
Three phases of water are shown in the figure.List the terms used to identify the phase changes indicated by the arrows. 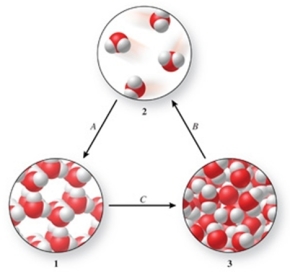
(Multiple Choice)
4.8/5  (39)
(39)
Rank the following substances in order of increasing boiling point: Cl2, Ar, Ne, Br2
(Multiple Choice)
4.7/5  (42)
(42)
Showing 21 - 40 of 107
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)