Exam 12: Radical Reactions
Exam 1: Atoms and Molecules; Orbitals and Bonding64 Questions
Exam 2: Alkanes65 Questions
Exam 3: Alkenes and Alkynes70 Questions
Exam 4: Stereochemistry68 Questions
Exam 5: Rings60 Questions
Exam 6: Substituted Alkanes: Alkyl Halides, Alcohols, Amines, Ethers,thiols, and Thioethers68 Questions
Exam 7: Substitution Reactions: the Sn2 and Sn1 Reactions55 Questions
Exam 8: Elimination Reactions: the E1 and E2 Reactions45 Questions
Exam 9: Analytical Chemistry: Spectroscopy65 Questions
Exam 10: Electrophilic Additions to Alkenes68 Questions
Exam 11: More Additions to Bonds65 Questions
Exam 12: Radical Reactions65 Questions
Exam 13: Dienes and the Allyl System: 2p Orbitals in Conjugation68 Questions
Exam 14: Aromaticity66 Questions
Exam 15: Substitution Reactions of Aromatic Compounds68 Questions
Exam 16: Carbonyl Chemistry 1: Addition Reactions73 Questions
Exam 17: Carboxylic Acids66 Questions
Exam 18: Derivatives of Carboxylic Acids: Acyl Compounds68 Questions
Exam 19: Carbonyl Chemistry 2: Reactions at the Α Position71 Questions
Exam 20: Special Topic: Carbohydrates40 Questions
Exam 21: Special Topic: Bio-Organic Chemistry40 Questions
Exam 22: Special Topic: Amino Acids and Polyamino Acids Peptides and Proteins39 Questions
Exam 23: Special Topic: Reactions Controlled by Orbital Symmetry46 Questions
Exam 24: Special Topic: Intramolecular Reactions and Neighboring Group Participation40 Questions
Select questions type
Which of the indicated C-C bonds requires the least amount of energy to break homolytically?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
B
Which of the following monobrominated products forms fastest under the conditions shown?
Free
(Multiple Choice)
4.7/5  (42)
(42)
Correct Answer:
B
Which of the following compounds is the product of this reaction? 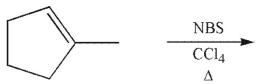
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
E
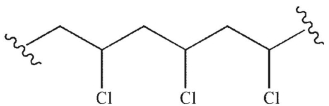
Draw all the constitutional isomers of the monochloroalkane generated from the free radical
chlorination of methylcyclohexane.Which of the constitutional isomers would also have
stereoisomers? 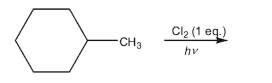
(Essay)
4.7/5  (36)
(36)
Which of the following monomers will react to provide the polymer shown here?
(Multiple Choice)
4.9/5  (43)
(43)
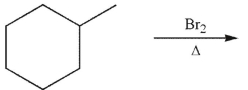
What is the principal monobrominated product that would result from the conditions shown? 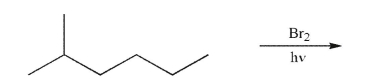
(Essay)
4.9/5  (32)
(32)
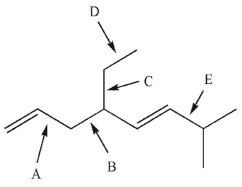
Devise a multistep synthesis of the target molecule from the given starting material.Show the
reagents needed for each step and the product of each step. 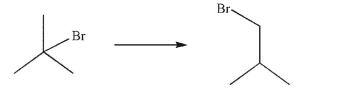
(Essay)
4.7/5  (42)
(42)
Which is the following would not be a valid resonance form for this radical?
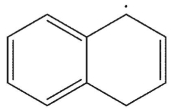
(Multiple Choice)
4.9/5  (31)
(31)
A chemist would like to prepare a tertiary alcohol via the following reaction sequence.
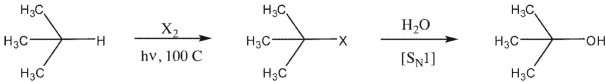 What halogen X should be used to ensure the maximum selectivity for the intermediate haloalkane?
What halogen X should be used to ensure the maximum selectivity for the intermediate haloalkane?
(Essay)
4.9/5  (35)
(35)
Anti-Markovnikov addition of HX to an alkene only works with HBr in the presence of peroxides
as a radical initiator.Use the bond dissociation data provided to explain why the reaction fails
with HCl and HI. 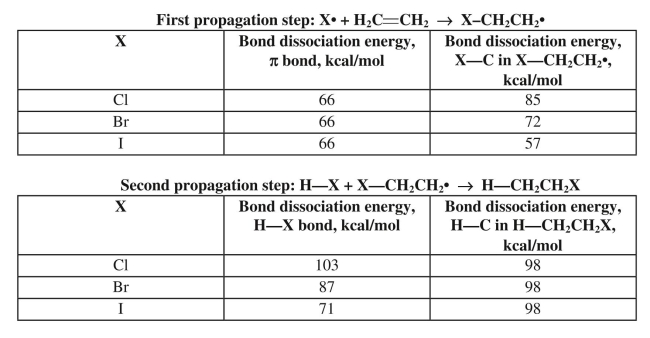
(Essay)
4.8/5  (36)
(36)
Which of the following transformations is a disproportionation?
(Multiple Choice)
4.8/5  (41)
(41)
Draw an arrow-pushing mechanism to show the transformation of dibromomethane to bromoform in
the presence of molecular bromine and bromine radical.
(Essay)
4.7/5  (40)
(40)
Devise a multistep synthesis of the target molecule from the given starting material.Show the
reagents needed for each step and the product of each step.Explain why the target cannot be made
from the starting material in one step. 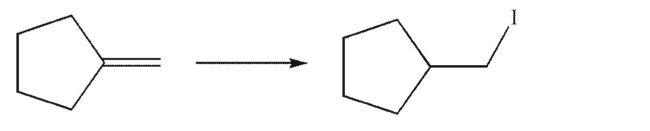
(Essay)
4.7/5  (31)
(31)
In an allylic bromination, why is it essential to keep the concentration of molecular bromine relatively low?
(Essay)
4.9/5  (36)
(36)
Using the bond dissociation energies provided below, draw an energy diagram for the two
propagation steps in the following reaction.  Show the correct relative energies of intermediates, starting materials and products. Estimate the
Show the correct relative energies of intermediates, starting materials and products. Estimate the 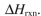 Bond dissociation energies (kcal/mol)
Bond dissociation energies (kcal/mol)
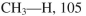
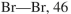
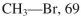
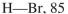
(Essay)
4.7/5  (31)
(31)
Provide the structures of all monochlorinated products that would result from the conditions
shown.Assume that all chiral products are formed as racemic mixtures. 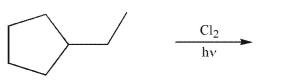
(Essay)
4.8/5  (19)
(19)
The disproportionation product(s) of the alkyl radical shown here will be 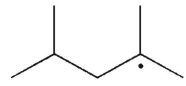
(Multiple Choice)
4.9/5  (36)
(36)
Showing 1 - 20 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)