Exam 16: Carbonyl Chemistry 1: Addition Reactions
Exam 1: Atoms and Molecules; Orbitals and Bonding64 Questions
Exam 2: Alkanes65 Questions
Exam 3: Alkenes and Alkynes70 Questions
Exam 4: Stereochemistry68 Questions
Exam 5: Rings60 Questions
Exam 6: Substituted Alkanes: Alkyl Halides, Alcohols, Amines, Ethers,thiols, and Thioethers68 Questions
Exam 7: Substitution Reactions: the Sn2 and Sn1 Reactions55 Questions
Exam 8: Elimination Reactions: the E1 and E2 Reactions45 Questions
Exam 9: Analytical Chemistry: Spectroscopy65 Questions
Exam 10: Electrophilic Additions to Alkenes68 Questions
Exam 11: More Additions to Bonds65 Questions
Exam 12: Radical Reactions65 Questions
Exam 13: Dienes and the Allyl System: 2p Orbitals in Conjugation68 Questions
Exam 14: Aromaticity66 Questions
Exam 15: Substitution Reactions of Aromatic Compounds68 Questions
Exam 16: Carbonyl Chemistry 1: Addition Reactions73 Questions
Exam 17: Carboxylic Acids66 Questions
Exam 18: Derivatives of Carboxylic Acids: Acyl Compounds68 Questions
Exam 19: Carbonyl Chemistry 2: Reactions at the Α Position71 Questions
Exam 20: Special Topic: Carbohydrates40 Questions
Exam 21: Special Topic: Bio-Organic Chemistry40 Questions
Exam 22: Special Topic: Amino Acids and Polyamino Acids Peptides and Proteins39 Questions
Exam 23: Special Topic: Reactions Controlled by Orbital Symmetry46 Questions
Exam 24: Special Topic: Intramolecular Reactions and Neighboring Group Participation40 Questions
Select questions type
Rank these carbonyl compounds in order of increasing C=O dipole moment.
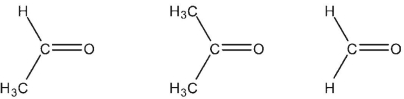
Free
(Essay)
4.7/5  (50)
(50)
Correct Answer:

How would you accomplish the following oxidation reaction? 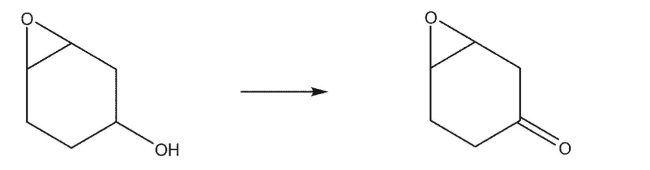
Free
(Essay)
4.9/5  (32)
(32)
Correct Answer:
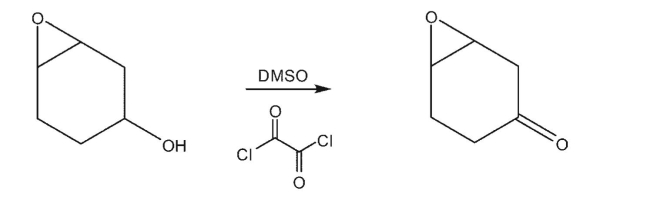
Which of the following is the major product of the reaction conditions shown?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
C
Which reagents would you use to accomplish the following transformation?
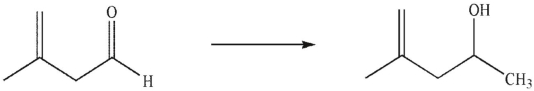
(Multiple Choice)
4.9/5  (39)
(39)
Design a multistep synthesis for the transformation shown here.You may use any organic or inorganic reagents.Show the reagents needed for each step and the product of each step. 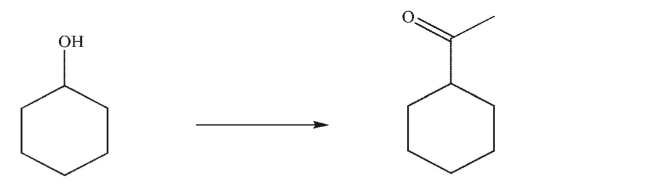
(Essay)
4.8/5  (27)
(27)
What are the missing reagents in the reaction sequence shown here?
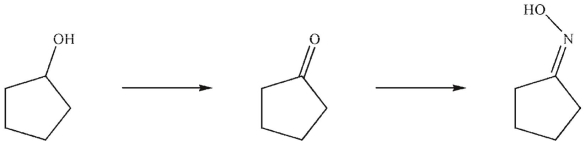
(Multiple Choice)
5.0/5  (45)
(45)
Which of the following compounds will not react with CrO3/pyridine to give a carbonyl compound?
(Multiple Choice)
4.8/5  (39)
(39)
In the multistep synthesis shown here, what would be the first step?
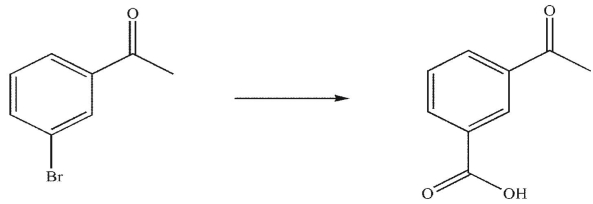
(Multiple Choice)
4.8/5  (36)
(36)
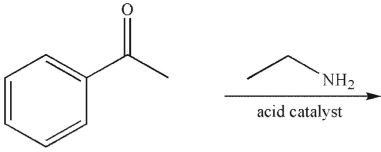
Which of the following compounds cannot be used to form an imine?
(Multiple Choice)
5.0/5  (34)
(34)
Draw a mechanism to illustrate the removal of the acetal under the conditions shown.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 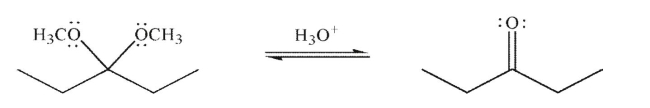
(Essay)
4.8/5  (40)
(40)
An acyclic compound was treated with trace acid to produce this structure. 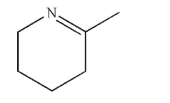 Draw the structure of the acyclic compound.
Draw the structure of the acyclic compound.
(Essay)
4.7/5  (42)
(42)
Draw a mechanism for the hydration of the compound shown here under acidic conditions. Include all lone pairs, curved arrows, and nonzero formal charges. 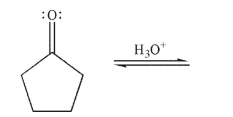
(Essay)
4.8/5  (35)
(35)
Which of the following statements about the biological oxidation of alcohols is false?
(Multiple Choice)
4.9/5  (36)
(36)
Predict the product of the following reaction and draw a mechanism to rationalize its formation. Include all necessary lone pairs, curved arrows, and nonzero formal charges. 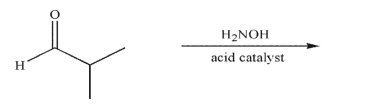
(Essay)
4.9/5  (33)
(33)
Which carbonyl compounds below has the IUPAC name: (2 R, 3 S)-2,3 -dibromo-4-methylpentanal?
(Multiple Choice)
4.7/5  (30)
(30)
Use a retrosynthetic analysis to provide five possible precursors to this alcohol. Include the reagents needed to transform each of the precursors to the alcohol. 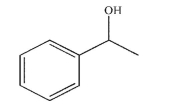
(Essay)
4.8/5  (35)
(35)
Draw a mechanism for the hydration of the compound shown here under basic conditions.Include
all lone pairs, curved arrows, and nonzero formal charges. 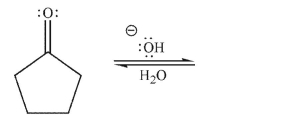
(Essay)
4.9/5  (43)
(43)
Showing 1 - 20 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)