Exam 2: The Chemical Level of Organization
Exam 1: An Introduction to Anatomy and Physiology123 Questions
Exam 2: The Chemical Level of Organization106 Questions
Exam 3: Cell Structure and Function126 Questions
Exam 4: The Tissue Level of Organization87 Questions
Exam 5: The Integumentary System85 Questions
Exam 6: The Skeletal System188 Questions
Exam 7: The Muscular System115 Questions
Exam 8: The Nervous System215 Questions
Exam 9: The General and Special Senses118 Questions
Exam 10: The Endocrine System100 Questions
Exam 11: The Cardiovascular System: Blood80 Questions
Exam 12: The Cardiovascular System: the Heart115 Questions
Exam 13: The Cardiovascular System: Blood Vessels and Circulation121 Questions
Exam 14: The Lymphoid System and Immunity105 Questions
Exam 15: The Respiratory System101 Questions
Exam 16: The Digestive System129 Questions
Exam 17: Nutrition and Metabolism78 Questions
Exam 18: The Urinary System117 Questions
Exam 19: The Reproductive System122 Questions
Exam 20: Development and Inheritance97 Questions
Select questions type
A decrease of hydrogen ions in the body fluids can have disastrous results because
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
E
If an element is composed of atoms with an atomic number of 8 and a mass number of 14, then a neutral atom of this element contains
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
B
Chemical reactions that occur in the human body are controlled by special protein molecules called _________________________.
Free
(Short Answer)
4.8/5  (33)
(33)
Correct Answer:
enzymes
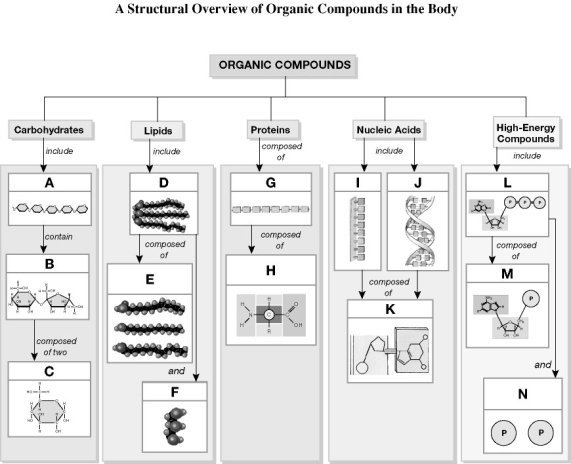 Using the figure above, identify the labeled part.
-Label L: ________
Using the figure above, identify the labeled part.
-Label L: ________
(Short Answer)
4.8/5  (34)
(34)
Which of the following is a weak electrical attraction between molecules?
(Multiple Choice)
4.8/5  (24)
(24)
 Using the figure above, identify the labeled part.
-Label H: ________
Using the figure above, identify the labeled part.
-Label H: ________
(Short Answer)
4.9/5  (30)
(30)
A class of lipids used to signal cells to undergo changes is called
(Multiple Choice)
4.9/5  (37)
(37)
 Using the figure above, identify the labeled part.
-Label J: ________
Using the figure above, identify the labeled part.
-Label J: ________
(Short Answer)
4.7/5  (36)
(36)
_________________________ are soluble inorganic compounds whose ions will conduct an electric current in solutions.
(Short Answer)
4.9/5  (28)
(28)
How many electrons do MOST atoms need in their outer shell to be stable?
(Multiple Choice)
4.7/5  (35)
(35)
Which statement about the reaction H2 + Cl2 → 2HCl is correct?
(Multiple Choice)
4.7/5  (28)
(28)
A solution containing more hydrogen ions than hydroxide ions is
(Multiple Choice)
4.8/5  (31)
(31)
All of the elements and compounds that are eaten and used by the body for some function are called
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following is a characteristic of hydrogen bonds?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)