Exam 24: Nonmetallic Elements and Their Compounds
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which of these reactions represents the removal of silica from iron ore in a blast furnace?
(Multiple Choice)
4.9/5  (34)
(34)
Which diagram best corresponds to the energy level diagram of a semiconductor?
(Multiple Choice)
4.9/5  (43)
(43)
In the production of aluminum, bauxite is heated with sodium hydroxide solution to ________.
(Multiple Choice)
4.8/5  (33)
(33)
The Hall process involves the reduction of Al2O3 to aluminum by
(Multiple Choice)
4.8/5  (43)
(43)
A mineral deposit concentrated enough to allow economical recovery of a desired metal is known as ________.
(Short Answer)
4.8/5  (39)
(39)
In gunpowder, which component is present in the highest percent by mass?
(Multiple Choice)
4.7/5  (29)
(29)
The process used to produce metals with a purity of more than 99.99% is called
(Multiple Choice)
4.9/5  (39)
(39)
Which reaction is a good small-scale laboratory method for the preparation of hydrogen?
(Multiple Choice)
4.8/5  (36)
(36)
The first step in the purification of a metal M is represented by the following figure. To which process does the figure correspond, and which metal is usually purified by this process? 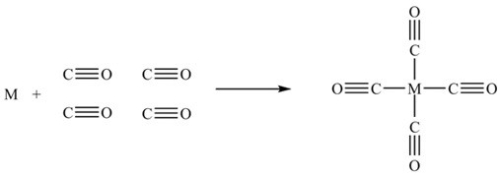
(Multiple Choice)
4.9/5  (32)
(32)
Beryllium, the first element in Group 2A, is the most metallic element in the group.
(True/False)
4.9/5  (35)
(35)
What is the science and technology of separating metals from their ores and of compounding alloys?
(Short Answer)
4.8/5  (30)
(30)
Which of these ions is most likely to substitute for Ca2+ in the human body?
(Multiple Choice)
4.7/5  (30)
(30)
Seawater is a good source of which of the following metals?
(Multiple Choice)
4.7/5  (35)
(35)
The following electrolytic cell is a representation of the process used to purify copper metal. How does it work? 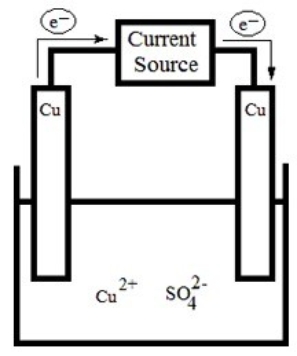
(Multiple Choice)
4.8/5  (40)
(40)
Mercury, magnesium, and zinc have low enough boiling points that they can be purified by distillation.
(True/False)
4.7/5  (43)
(43)
Showing 41 - 60 of 117
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)