Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Liquid sodium can be used as a heat transfer fluid. Its vapor pressure is 40.0 torr at 633°C and 400.0 torr at 823°C. What is its heat of vaporization?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
D
Which of the following properties indicates the presence of strong intermolecular forces in a liquid?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
________ is the name given to the phase change from a solid directly to a gas.
Free
(Short Answer)
4.8/5  (41)
(41)
Correct Answer:
Sublimation
Molecular crystals are held together by the intermolecular forces of dispersion and dipole-dipole forces and by hydrogen bonding.
(True/False)
5.0/5  (37)
(37)
What is determined by the magnitude of intermolecular forces in a liquid and is a measure of a fluid's resistance to flow?
(Multiple Choice)
4.7/5  (39)
(39)
What is defined as the number of atoms surrounding an atom in a crystal lattice and indicates how tightly the atoms are packed together?
(Multiple Choice)
4.8/5  (42)
(42)
________ ________ are characterized by an instantaneous dipole.
(Short Answer)
4.9/5  (38)
(38)
What name is given to the curved surface of a liquid contained in a narrow tube?
(Multiple Choice)
4.9/5  (37)
(37)
The shape of the water-to-glass meniscus results from the strong adhesive forces between glass and water.
(True/False)
4.8/5  (39)
(39)
Based on the phase diagram of a pure substance given below, which statement is true? 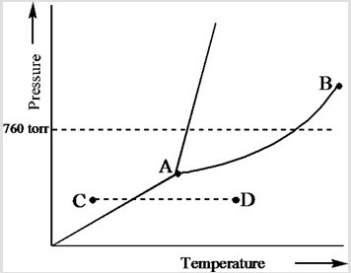
(Multiple Choice)
4.9/5  (50)
(50)
Based on the phase diagram of a pure substance given below, what is the significance of the point labeled B? 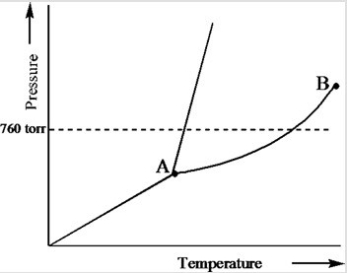
(Multiple Choice)
4.9/5  (33)
(33)
Based on the phase diagram of a pure substance given below, what change of state occurs as the substance changes from point C to point D? 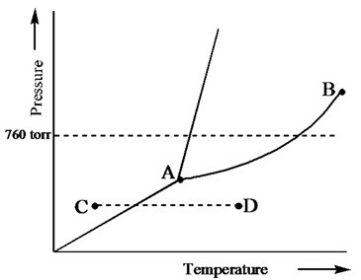
(Multiple Choice)
4.8/5  (35)
(35)
Which is the correct equation for the molar heat of sublimation?
(Multiple Choice)
4.8/5  (42)
(42)
Which one of the following crystallizes in a metallic lattice?
(Multiple Choice)
4.8/5  (38)
(38)
In a sample of hydrogen iodide, ________ are the most important intermolecular forces.
(Multiple Choice)
4.9/5  (39)
(39)
Identify the dominant (strongest) type of intermolecular force present in Cl2(l).
(Short Answer)
4.9/5  (42)
(42)
The energy required to increase the surface of a liquid per unit area is called the
(Multiple Choice)
4.7/5  (30)
(30)
How many phase changes are represented in the following heating curve of a pure substance? 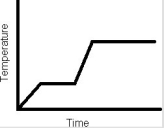
(Multiple Choice)
4.8/5  (45)
(45)
Showing 1 - 20 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)