Exam 2: Atoms, Molecules, and Ions
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which of the following is the oxoanion of bromine called the bromate ion?
Free
(Multiple Choice)
5.0/5  (37)
(37)
Correct Answer:
A
In the early 1900s, Ernest Rutherford performed an experiment with thin foils of gold and alpha particles to probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have equal numbers of protons and electrons) and that the mass of a proton is significantly greater than the mass of an electron, use Rutherford's data to propose a structural model of an atom.
Free
(Essay)
4.9/5  (38)
(38)
Correct Answer:
(Answers will vary.) Atoms are mostly empty space. The mass is concentrated mostly at the center of the atom.
Which of these compounds is most likely to be ionic?
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
A
Which of these scientists developed the nuclear model of the atom?
(Multiple Choice)
4.8/5  (35)
(35)
Which field of study made a big contribution toward understanding the composition of the atom?
(Multiple Choice)
4.7/5  (39)
(39)
State the two important experimental results (and the names of the responsible scientists) which enabled the mass of the electron to be determined.
(Essay)
4.9/5  (33)
(33)
Many different compounds might be represented by the same empirical formula.
(True/False)
4.7/5  (31)
(31)
Who is credited with first measuring the charge of the electron?
(Multiple Choice)
4.9/5  (42)
(42)
Lithium forms compounds which are used in dry cells, storage batteries, and in high-temperature lubricants. It has two naturally occurring isotopes, 6Li (isotopic mass = 6.015123 amu) and 7Li (isotopic mass = 7.016005 amu). Lithium has an atomic mass of 6.9412 amu. What is the percent abundance of lithium-6?
(Multiple Choice)
4.9/5  (35)
(35)
What element is represented by X in the atomic symbol notation 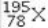 ?
?
(Multiple Choice)
4.8/5  (30)
(30)
Ionic compounds may carry a net positive or net negative charge.
(True/False)
4.8/5  (44)
(44)
Which of the following is a type of radioactive radiation that consists of positively charged particles and is deflected away from the positively charged plate?
(Multiple Choice)
4.9/5  (37)
(37)
What name is given to the simplest organic compounds which only contain carbons and hydrogens?
(Short Answer)
4.8/5  (43)
(43)
Silicon, which makes up about 25% of Earth's crust by mass, is used widely in the modern electronics industry. It has three naturally occurring isotopes, 28Si, 29Si, and 30Si. Calculate the atomic mass of silicon. 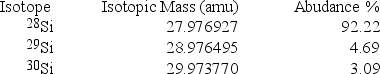
(Multiple Choice)
4.8/5  (33)
(33)
Why was it more difficult to design an experiment that would prove the existence of neutrons than it was to design an experiment that would prove the existence of either protons or electrons?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)