Exam 7: Electron Configuration and the Periodic Table
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which ground-state ion does not have an electron configuration described by the following orbital diagram? 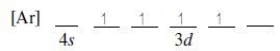
(Multiple Choice)
4.9/5  (35)
(35)
How does atomic radius change as you move across the periodic table?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the species listed below is not isoelectronic with the others?
(Multiple Choice)
4.9/5  (33)
(33)
Explain why the trend in electron affinity increases in general as you move from left to right across a period (row) of the periodic table.
(Essay)
4.8/5  (33)
(33)
How many protons and electrons are present in one Br- ion?
(Multiple Choice)
4.9/5  (37)
(37)
Which of these elements has the greatest metallic character?
(Multiple Choice)
4.9/5  (41)
(41)
Which element in Group 6A has the highest electron affinity?
(Multiple Choice)
4.8/5  (35)
(35)
Consider the set of isoelectronic atoms and ions A2-, B-, C, D+, and E2+. Which arrangement of relative radii is correct?
(Multiple Choice)
4.9/5  (44)
(44)
If the radius of atom X is greater than the radius of atom Y, then it is also likely that
(Multiple Choice)
4.8/5  (35)
(35)
The energy states of atoms containing more than one electron arise from nucleus-electron and electron-electron interactions. Which of the following statements correctly describes these effects?
(Multiple Choice)
4.7/5  (30)
(30)
In 1864, the English chemist John Newlands noticed that when the elements were arranged in order of increasing atomic mass, every eighth element had similar properties. Newlands referred to this relationship as the
(Multiple Choice)
4.8/5  (33)
(33)
Who noticed that the periodic table was arranged in order of atomic mass, where every eighth element had similar properties, and called this the law of octaves?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 101 - 120 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)