Exam 3: Stoichiometry: Ratios of Combination
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Suppose white atoms (W) and black atoms (B) react according to the following balanced chemical equation. 2W + B → BW2
If the initial reaction mixture is prepared as follows, what will the final reaction mixture be once the reaction is complete? 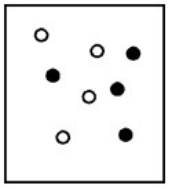
(Multiple Choice)
4.8/5  (27)
(27)
Ammonia reacts with diatomic oxygen to form nitric oxide and water vapor as follows: 4NH3 + 5O2 → 4NO + 6H2O
What is the maximum amount of water that may be produced if 40.0 g NH3 and 50.0 g O2 are mixed and allowed to react?
(Multiple Choice)
4.9/5  (31)
(31)
Terephthalic acid, used in the production of polyester fibers and films, is composed of carbon, hydrogen, and oxygen. When 0.6943 g of terephthalic acid was subjected to combustion analysis, it produced 1.471 g CO2 and 0.226 g H2O. What is its empirical formula?
(Multiple Choice)
5.0/5  (33)
(33)
What is the coefficient of H2O when the following equation is properly balanced with the smallest set of whole numbers? ________ Al4C3 + ________ H2O → ________ Al(OH)3 + ________ CH4
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the mass in grams of 8.35 × 1022 molecules of CBr4.
(Multiple Choice)
4.7/5  (36)
(36)
Methanol (CH3OH) is converted to bromomethane (CH3Br) as follows: CH3OH + HBr → CH3Br + H2O
If 12.23 g of bromomethane are produced when 5.00 g of methanol is reacted with excess HBr, what is the percentage yield?
(Multiple Choice)
4.7/5  (47)
(47)
What is the coefficient of H2O when the following equation is properly balanced with the smallest set of whole numbers? ________ Na + ________ H2O → ________ NaOH + ________ H2
(Multiple Choice)
4.8/5  (39)
(39)
Tetraphosphorus hexaoxide is formed by the reaction of phosphorus with oxygen gas. If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
(Multiple Choice)
4.8/5  (40)
(40)
Potassium chloride is used as a substitute for sodium chloride for individuals with high blood pressure. Identify the limiting reactant and determine the mass of the excess reactant remaining when 7.00 g of chlorine gas reacts with 5.00 g of potassium to form potassium chloride.
(Multiple Choice)
4.8/5  (42)
(42)
Which could represent the reaction mixture after the complete combustion of acetaldehyde, CH3CHO?
(Multiple Choice)
4.8/5  (38)
(38)
How many grams are contained in a 0.893 mol sample of methane, CH4?
(Multiple Choice)
4.9/5  (49)
(49)
What mass of FeS is formed if 9.42 g of Fe reacts with 68.0 g of S8? 8Fe(s) + S8(s) → 8FeS(s)
(Multiple Choice)
4.8/5  (41)
(41)
Aluminum sulfate, Al2(SO4)3, is used in tanning leather, purifying water, and the manufacture of antiperspirants. Calculate its formula mass.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 121 - 137 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)