Exam 2: Atoms, Molecules, and Ions
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
Which of the following is a molecular formula for a compound with an empirical formula of CH?
(Multiple Choice)
4.8/5  (36)
(36)
Potassium permanganate is a strong oxidizer that reacts explosively with easily oxidized materials. What is its formula?
(Multiple Choice)
4.8/5  (28)
(28)
Rutherford's experiment with alpha particle scattering by gold foil established that
(Multiple Choice)
4.8/5  (40)
(40)
What binary compound would be formed from barium ions and fluoride ions?
(Multiple Choice)
4.9/5  (36)
(36)
Tetrasulfur dinitride decomposes explosively when heated. What is its formula?
(Multiple Choice)
5.0/5  (45)
(45)
Who is credited with measuring the mass/charge ratio of the electron?
(Multiple Choice)
4.7/5  (33)
(33)
Atoms X, Y, Z, and R have the following nuclear compositions: 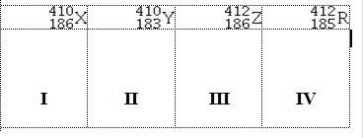 Which of the following are isotopes of the same element?
Which of the following are isotopes of the same element?
(Multiple Choice)
4.8/5  (28)
(28)
What is the formula for the ionic compound formed by calcium and selenium?
(Multiple Choice)
4.8/5  (36)
(36)
Determine the number of electrons and identify the correct symbol for an atom with 17 protons and 18 neutrons.
(Multiple Choice)
4.8/5  (39)
(39)
What is the formula for the ionic compound formed by calcium ions and nitrate ions?
(Multiple Choice)
4.9/5  (43)
(43)
The molecular formula of a molecular substance is a whole number multiple of its empirical formula.
(True/False)
4.8/5  (34)
(34)
The chemical name for SO32- (aq) is sulfite ion. Therefore, the chemical name of H2SO3 (aq) is
(Multiple Choice)
4.9/5  (39)
(39)
Showing 21 - 40 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)