Exam 6: Properties of Solutions I: Aqueous Solutions
Exam 1: Introduction to Forensic Chemistry20 Questions
Exam 2: Evidence Collection and Preservation19 Questions
Exam 3: Atomic Clues19 Questions
Exam 4: Chemical Evidence25 Questions
Exam 5: Chemistry of Bonding:20 Questions
Exam 6: Properties of Solutions I: Aqueous Solutions20 Questions
Exam 7: Properties of Solutions Ii: Intermolecular Forces and Colligative Properties24 Questions
Exam 8: Drug Chemistry25 Questions
Exam 9: Chemistry of Fire and Heat19 Questions
Exam 10: Chemistry of Explosions22 Questions
Exam 11: Applications of Chemical Kinetics20 Questions
Exam 12: Nuclear Chemistry: Energy, Medicine, Weapons, and Terrorism22 Questions
Exam 13: Chemical Equilibrium and Poisons20 Questions
Exam 14: Introduction to Biochemistry and Dna Analysis20 Questions
Select questions type
State whether each of the following compounds is an electrolyte or nonelectrolyte.
A) CaCl2 ______
B) CO2 _____
C) H2SO4 _____
D) C6H12O6 _____
Free
(Essay)
4.8/5  (32)
(32)
Correct Answer:
electrolyte; nonelectrolyte; electrolyte; nonelectrolyte
Write the net ionic equation for the precipitation of lead(II) iodide from the addition of lead(II) nitrate solution to a potassium iodide solution.
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
Pb2+(aq) + 2I-(aq) → PbI2(s)
Which of the following compounds is a strong electrolyte?
Free
(Multiple Choice)
4.9/5  (47)
(47)
Correct Answer:
C
Identify H2CO3 as a strong acid, strong base, weak acid, or weak base.
(Multiple Choice)
4.8/5  (33)
(33)
Triclosan is an antibacterial agent present in some soaps and surgical cleaning treatments. Calculate the mass of triclosan, C12H7Cl3O2, needed to prepare 750.0 mL of a 5.00 × 10-5 M solution.
(Multiple Choice)
4.9/5  (42)
(42)
Hydrofluoric acid is used to etch glass. Concentrated hydrofluoric acid is 28.9 M. If a 500.0-mL sample of 5.75 M HF is needed, how many mL of the concentrated HF should be diluted?
(Multiple Choice)
4.8/5  (39)
(39)
A luminol solution is used to detect the presence of blood. What is the molarity of a luminol solution that was made by dissolving 15.5 g of luminol (C8H7N3O2) in water to a final volume of 90.0 mL?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is the correct name for the acid H3PO4?
(Multiple Choice)
4.9/5  (47)
(47)
Which term is used to describe a solution that contains less solute than the maximum amount of solute that can be dissolved?
(Multiple Choice)
4.8/5  (38)
(38)
What is the molarity of a solution prepared by dissolving 29.3 g KCl in water to a final volume of 500.0 mL?
(Multiple Choice)
4.9/5  (36)
(36)
What ion would be used to form an insoluble compound with the ion Hg22+?
(Multiple Choice)
4.9/5  (37)
(37)
Identify Ca(OH)2 as a strong acid, strong base, weak acid, or weak base.
(Multiple Choice)
4.8/5  (32)
(32)
An unknown solution is determined to have a pH of 4.5. Identify the solution as being acidic, basic, or neutral.
(Multiple Choice)
4.8/5  (32)
(32)
A 25.0-mL solution contains 0.45 g of cadmium cyanide. Which type of solution is this?
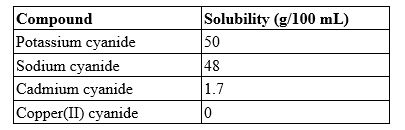
(Multiple Choice)
4.7/5  (39)
(39)
A 100.0-mL solution contains 27.8 g of sodium cyanide. Which type of solution is this?
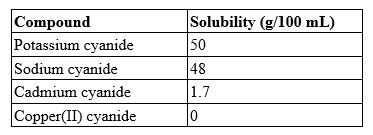
(Multiple Choice)
4.8/5  (26)
(26)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)