Exam 6: Ionic and Molecular Compounds
Exam 1: Chemistry in Our Lives40 Questions
Exam 2: Chemistry and Measurements70 Questions
Exam 3: Matter and Energy77 Questions
Exam 4: Atoms and Elements83 Questions
Exam 5: Nuclear Chemistry64 Questions
Exam 6: Ionic and Molecular Compounds83 Questions
Exam 7: Chemical Reactions and Quantities96 Questions
Exam 8: Gases84 Questions
Exam 9: Solutions84 Questions
Exam 10: Reaction Rates and Chemical Equilibrium52 Questions
Exam 11: Acids and Bases72 Questions
Exam 12: Introduction to Organic Chemistry: Hydrocarbons105 Questions
Exam 13: Alcohols, Phenols, Thiols, and Ethers49 Questions
Exam 14: Aldehydes and Ketones44 Questions
Exam 15: Carbohydrates54 Questions
Exam 16: Carboxylic Acids and Esters55 Questions
Exam 17: Lipids70 Questions
Exam 18: Amines and Amides60 Questions
Exam 19: Amino Acids and Proteins66 Questions
Exam 20: Enzymes and Vitamins68 Questions
Exam 21: Nucleic Acids and Protein Synthesis55 Questions
Exam 22: Metabolic Pathways for Carbohydrates58 Questions
Exam 23: Metabolism and Energy Production51 Questions
Exam 24: Metabolic Pathways for Lipids and Amino Acids56 Questions
Select questions type
The number of valence electrons found in an atom of a Group A element is equal to
(Multiple Choice)
4.8/5  (37)
(37)
The strongest interactions between molecules of iodine, 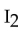 , are examples of
, are examples of
(Multiple Choice)
4.9/5  (34)
(34)
The strongest interactions between atoms of helium, He, are examples of
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following polyatomic ions has a positive charge?
(Multiple Choice)
4.8/5  (34)
(34)
The strongest interactions in the compound sodium fluoride, NaF, are examples of
(Multiple Choice)
4.8/5  (39)
(39)
In a covalently bonded molecule, the number of electrons that an atom shares with others is usually equal to the number of electrons
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following elements has the lowest electronegativity?
(Multiple Choice)
4.7/5  (42)
(42)
A polar covalent bond is found in which of these compounds?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following substances contains a nonpolar covalent bond?
(Multiple Choice)
4.9/5  (37)
(37)
A(n)________ is the smallest unit of two or more atoms held together by a covalent bond.
(Multiple Choice)
4.9/5  (32)
(32)
Showing 41 - 60 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)