Exam 11: Chemical Kinetics
Exam 1: Introduction to Chemistry42 Questions
Exam 2: Atoms and Molecules45 Questions
Exam 3: Molecules Moles Chemical Equations45 Questions
Exam 4: Stoichiometry40 Questions
Exam 5: Gases45 Questions
Exam 6: The Periodic Table and Atomic Structure45 Questions
Exam 7: Chemical Bonding and Molecular Structure40 Questions
Exam 8: Molecules and Materials37 Questions
Exam 9: Energy and Chemistry40 Questions
Exam 10: Entropy and the Second Law of Thermodynamics30 Questions
Exam 11: Chemical Kinetics40 Questions
Exam 12: Chemical Equilibrium40 Questions
Exam 13: Electrochemistry40 Questions
Select questions type
The radioactive nuclide 14C decays with a t1/2 of 5730 yrs. An archaeologist is studying a woven mat using radiocarbon dating. If calculations related to the mat's original 14C content reveal that there was 5.83 × 1022 atoms of 14C present in the plant used to weave the mat, how long ago was the mat woven if modern spectroscopy was used to determine that 7.11 × 1020 atoms of 14C now exist in the sample?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
Consider the reaction: A + 2B → C,
And a kinetics study on this reaction yielded:
![Consider the reaction: A + 2B → C, And a kinetics study on this reaction yielded: What order is the reaction with respect to ([A], [B])?](https://storage.examlex.com/TBX8709/11ebe889_6d7f_39f8_b264_37d48e692754_TBX8709_00.jpg) What order is the reaction with respect to ([A], [B])?
What order is the reaction with respect to ([A], [B])?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
A
According to the differential rate law: rate = k[X]m[Y]n. If m = 2 and n = 1, the overall reaction order is:
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
A
The synthesis of ammonia from hydrogen and nitrogen produced the following kinetic data:
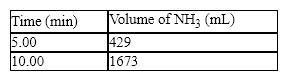 What is the average rate in mL/min for the first five minutes of the reaction?
What is the average rate in mL/min for the first five minutes of the reaction?
(Multiple Choice)
4.8/5  (44)
(44)
If a reaction is second order with respect to [A], doubling the concentration of [A] will result in:
(Multiple Choice)
4.9/5  (32)
(32)
Consider the elementary step: A + B → C. What type of elementary step is this?
(Multiple Choice)
4.8/5  (33)
(33)
For a first-order reaction, a plot of concentration vs time provides a line with a slope equal to − k.
(True/False)
4.7/5  (32)
(32)
Consider the reaction: 2A + B → C,
And a kinetics study on this reaction yielded:
![Consider the reaction: 2A + B → C, And a kinetics study on this reaction yielded: What order is the reaction with respect to ([A], [B])?](https://storage.examlex.com/TBX8709/11ebe889_6d7f_6109_b264_a9f7e71db46a_TBX8709_00.jpg) What order is the reaction with respect to ([A], [B])?
What order is the reaction with respect to ([A], [B])?
(Multiple Choice)
4.9/5  (42)
(42)
The production of nitric oxide is governed by the reaction:
4 NH3( g ) + 5 O2( g ) → 4 NO( g ) + 6 H2O( g )
If the rate at which NO is produced is 6.35 × 10 −2 mol L − 1s − 1, at what rate is O2 consumed?
(Multiple Choice)
4.7/5  (36)
(36)
Consider the production of oxygen from ozone. The mechanism includes two elementary steps:
O3 + Cl → ClO + O2 ClO + O3 → Cl + 2O2
Which species is a reactive intermediate?
(Multiple Choice)
4.8/5  (36)
(36)
For a zero order reaction, a plot of concentration with respect to time produces a plot that is:
(Multiple Choice)
4.9/5  (27)
(27)
If a reaction is first order with respect to [A], doubling the concentration of [A] will result in:
(Multiple Choice)
4.7/5  (41)
(41)
If a reaction is zero order with respect to [B], tripling the concentration of [B] will result in:
(Multiple Choice)
4.7/5  (44)
(44)
Stirring a reaction increases the rate of the reaction by raising the energy of activation.
(True/False)
4.9/5  (43)
(43)
The rate of a reaction may be expressed as the disappearance of reactants with respect to time.
(True/False)
4.9/5  (30)
(30)
The instantaneous rate of a reaction is determined using the slope of a line tangent to the curve of the change of concentration versus time.
(True/False)
4.9/5  (32)
(32)
During the formation of MgO from the burning of Mg metal, what steps will result in an increase in the rate of reaction?
(Multiple Choice)
5.0/5  (33)
(33)
Showing 1 - 20 of 40
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)