Exam 12: Chemical Equilibrium
Exam 1: Introduction to Chemistry42 Questions
Exam 2: Atoms and Molecules45 Questions
Exam 3: Molecules Moles Chemical Equations45 Questions
Exam 4: Stoichiometry40 Questions
Exam 5: Gases45 Questions
Exam 6: The Periodic Table and Atomic Structure45 Questions
Exam 7: Chemical Bonding and Molecular Structure40 Questions
Exam 8: Molecules and Materials37 Questions
Exam 9: Energy and Chemistry40 Questions
Exam 10: Entropy and the Second Law of Thermodynamics30 Questions
Exam 11: Chemical Kinetics40 Questions
Exam 12: Chemical Equilibrium40 Questions
Exam 13: Electrochemistry40 Questions
Select questions type
The term "strong acid" refers to any acid of 0.100 M concentration or higher.
Free
(True/False)
4.9/5  (34)
(34)
Correct Answer:
False
What is the pH of a 0.150 M NaOH solution?
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
D
What change in reaction direction occurs if dilute HCl is added to a H2PO4 − solution?
H2PO4 1− + H2O 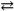 HPO42 − + H3O+
HPO42 − + H3O+
Free
(Multiple Choice)
4.7/5  (28)
(28)
Correct Answer:
B
According to the Brønsted-Lowry theory of acids and bases, acids are proton (hydronium) acceptors.
(True/False)
4.9/5  (41)
(41)
The synthesis of the pesticide thionyl chloride (OSCl2) proceeds according to:
SO3( g ) + SCl2( l ) 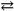 OSCl2( l ) + SO2( g )
If Δ G0 = − 106 kJ/mol for this reaction at 298K, what is the Keq at this temperature? Recall: R = 8.314 J mol − 1 K − 1.
OSCl2( l ) + SO2( g )
If Δ G0 = − 106 kJ/mol for this reaction at 298K, what is the Keq at this temperature? Recall: R = 8.314 J mol − 1 K − 1.
(Multiple Choice)
4.8/5  (34)
(34)
Consider the weak base ClO − .
ClO − + H2O ![Consider the weak base ClO<sup> − </sup>. ClO<sup> − </sup> + H<sub>2</sub>O HClO + OH<sup> − </sup> If a dilute HCl is slowly titrated into a sample of ClO<sup> − </sup>, the [ClO<sup> − </sup>] concentration:](https://storage.examlex.com/TBX8709/11ebe889_6d80_e7b1_b264_253759d1b45c_TBX8709_11.jpg) HClO + OH −
If a dilute HCl is slowly titrated into a sample of ClO − , the [ClO − ] concentration:
HClO + OH −
If a dilute HCl is slowly titrated into a sample of ClO − , the [ClO − ] concentration:
(Multiple Choice)
4.8/5  (33)
(33)
A catalyst will increase the rate of the forward and reverse reactions equally.
(True/False)
4.8/5  (32)
(32)
A large "K" value (>0) indicates that the reaction tends to favor the forward direction and the formation of products.
(True/False)
4.9/5  (38)
(38)
If NaCl is added to a saturated solution of AgCl, the equilibrium will shift back towards the reactants; and the solubility of AgCl will decrease.
(True/False)
4.9/5  (35)
(35)
What is the pH of a 0.1055 M CH3COOH solution (Ka = 1.75 × 10 − 5)?
(Multiple Choice)
4.8/5  (31)
(31)
Exothermic reactions in equilibrium will favor the reactants if the system is cooled.
(True/False)
4.8/5  (33)
(33)
Which "K" value indicates a reaction most favoring the formation of products?
(Multiple Choice)
4.9/5  (32)
(32)
Cememt structures with many small cracks are more likely to fail than similar structure with fewer, larger cracks.
(True/False)
4.8/5  (39)
(39)
Showing 1 - 20 of 40
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)