Exam 13: Electrochemistry
Exam 1: Introduction to Chemistry42 Questions
Exam 2: Atoms and Molecules45 Questions
Exam 3: Molecules Moles Chemical Equations45 Questions
Exam 4: Stoichiometry40 Questions
Exam 5: Gases45 Questions
Exam 6: The Periodic Table and Atomic Structure45 Questions
Exam 7: Chemical Bonding and Molecular Structure40 Questions
Exam 8: Molecules and Materials37 Questions
Exam 9: Energy and Chemistry40 Questions
Exam 10: Entropy and the Second Law of Thermodynamics30 Questions
Exam 11: Chemical Kinetics40 Questions
Exam 12: Chemical Equilibrium40 Questions
Exam 13: Electrochemistry40 Questions
Select questions type
In the formation of iron (II) oxide, which species is being reduced?
Fe( s ) + O2( g ) → FeO( s )
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
D
In the decomposition of hydrogen peroxide, which atom is being reduced?
H2O2( aq ) → H2( g ) + O2( g )
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
A
Galvanic corrosion may occur only when two different metals are in contact with one another.
Free
(True/False)
4.8/5  (36)
(36)
Correct Answer:
True
Which phrase best describes the half-reaction as written?
Na+ + e − → Na
(Multiple Choice)
4.8/5  (48)
(48)
What is the E0 value for the Galvanic cell formed from these two half-reactions?
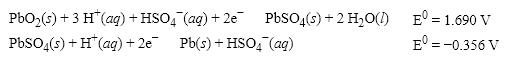
(Multiple Choice)
4.9/5  (37)
(37)
In the formation of iron (II) oxide, which species is being reduced?
Fe( s ) + O2( g ) → FeO( s )
(Multiple Choice)
4.8/5  (21)
(21)
Species with positive standard reduction potentials are excellent candidates for reduction reactions.
(True/False)
4.7/5  (35)
(35)
Balance the following electrochemical reaction in acid:
MnO4 − ( aq ) + Zr( s ) ↔ Mn2+( aq ) + Zr2+( aq )
(Multiple Choice)
4.7/5  (31)
(31)
What is the E0 value for the Galvanic cell formed from these two half-reactions?

(Multiple Choice)
4.9/5  (33)
(33)
The rusting witnessed in the sheet metal of a '65 Impala is an example of:
(Multiple Choice)
4.8/5  (29)
(29)
The standard state of an electrochemical cell is measured as 1 atm pressure for all gases and 1.0 M for all aqueous solutions.
(True/False)
4.8/5  (27)
(27)
Using the provided standard reduction potentials, which of the following cells is an example of a Galvanic cell?
(Multiple Choice)
4.7/5  (35)
(35)
How many grams of silver are deposited at a platinum cathode in the electrolysis of AgNO3 (aq) by 5.30 amps of electric current in 4.0 hours?
(Multiple Choice)
4.8/5  (32)
(32)
A Galvanic cell is an electrochemical cell in which a spontaneous chemical reaction may be used to generate an electrical current.
(True/False)
4.7/5  (33)
(33)
Given a Galvanic cell: 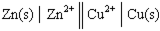 , the right-hand side of this notation represents the:
, the right-hand side of this notation represents the:
(Multiple Choice)
4.8/5  (41)
(41)
The standard hydrogen electrode (SHE) involves gaseous HCl at 1 atm being bubbled into water.
(True/False)
4.9/5  (39)
(39)
Showing 1 - 20 of 40
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)