Exam 6: The Periodic Table and Atomic Structure
Exam 1: Introduction to Chemistry42 Questions
Exam 2: Atoms and Molecules45 Questions
Exam 3: Molecules Moles Chemical Equations45 Questions
Exam 4: Stoichiometry40 Questions
Exam 5: Gases45 Questions
Exam 6: The Periodic Table and Atomic Structure45 Questions
Exam 7: Chemical Bonding and Molecular Structure40 Questions
Exam 8: Molecules and Materials37 Questions
Exam 9: Energy and Chemistry40 Questions
Exam 10: Entropy and the Second Law of Thermodynamics30 Questions
Exam 11: Chemical Kinetics40 Questions
Exam 12: Chemical Equilibrium40 Questions
Exam 13: Electrochemistry40 Questions
Select questions type
The ____________________ of a wave refers to the size or height of the wave.
Free
(Short Answer)
4.8/5  (29)
(29)
Correct Answer:
amplitude
The size of orbitals tends to decrease for large nuclei as a result of:
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
D
Which is not a legitimate value for the "n" quantum number?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
The "n" value of the quantum number assigned to an atom best describes the orbit's:
(Multiple Choice)
4.8/5  (29)
(29)
If a 755 nm photon travels in a vacuum, what is its frequency of this photon? Recall: the speed of light in a vacuum is 3.00 × 108 m/s.
(Multiple Choice)
4.7/5  (37)
(37)
The now-characteristic model of the atom relies upon the idea that the electrons reside in stable orbits from which they can not deviate and are found outside of a small, densely packed group of protons. This model was created by:
(Multiple Choice)
4.8/5  (28)
(28)
A photon with a frequency of 2.1 × 104 s − 1 has an energy of:
(Multiple Choice)
4.8/5  (39)
(39)
In quantum mechanics, an " l " value of 2 corresponds to a letter designation of:
(Multiple Choice)
4.8/5  (35)
(35)
If the binding energy of an electron is 6.41 × 10 − 19 J, what wavelength of photon is required to liberate it from the atom? Planck's constant = 6.626 × 10 − 34 J ⋅ s; c = 3.00 × 108 m/s
(Multiple Choice)
4.8/5  (28)
(28)
The ____________________ of any wave is the distance between corresponding points on adjacent waves.
(Short Answer)
4.8/5  (39)
(39)
In quantum mechanics, an " l " value of 0 corresponds to a letter designation of:
(Multiple Choice)
4.7/5  (32)
(32)
The energy of an incoming photon must be greater than the binding energy of the metal's electron in order to have that electron ejected from the metal's surface.
(True/False)
4.9/5  (38)
(38)
Because the speed of light remains constant for a given media frequency and wavelength are inversely proportional.
(True/False)
4.8/5  (36)
(36)
The ____________________ Effect describes the ejection of an electron from the surface of an easily ionizable metal when a sufficiently energetic electron strikes that metal.
(Short Answer)
4.8/5  (33)
(33)
The bending of light as it passes between air and another denser media is referred to as:
(Multiple Choice)
4.7/5  (33)
(33)
The Pauli Exclusion Principle states that no two electrons in the same atom may have the same set of four quantum numbers.
(True/False)
4.8/5  (29)
(29)
If photon "A" has twice the energy of photon "B" in a given media, then "A" should have 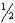 the frequency of "B".
the frequency of "B".
(True/False)
4.8/5  (32)
(32)
X-ray fluorescence is an analytical technique that uses high energy x-rays to eject protons from the nucleus of an atom.
(True/False)
4.9/5  (40)
(40)
Showing 1 - 20 of 45
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)