Exam 12: Organic Acids and Bases
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
Consider the structures given below. 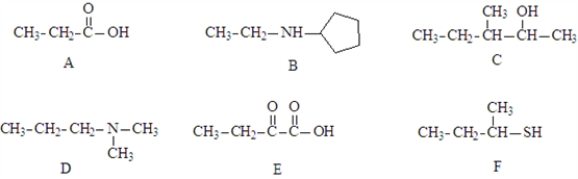 Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure _________________________ would undergo oxidative decarboxylation.
Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure _________________________ would undergo oxidative decarboxylation.
Free
(Short Answer)
4.8/5  (37)
(37)
Correct Answer:
D
Which of the following is characteristic of the structure of tryptamines?
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
D
Consider the structures given below.  Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure ___________________ would exist as a positive ion at pH 4.
Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure ___________________ would exist as a positive ion at pH 4.
Free
(Short Answer)
5.0/5  (43)
(43)
Correct Answer:
B
D
Which of the following is the molecular formula for the salt of the magnesium ion and the propanoate anion?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is true of both a decarboxylation reaction and an oxidative decarboxylation reaction?
(Multiple Choice)
4.8/5  (29)
(29)
Using structural formulas, write the equation for the ionization of p-cresol (show below) in water. 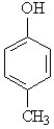
(Essay)
4.9/5  (37)
(37)
In the synthesis of terpenes, an important class of biomolecules called lipids, mevalonyl phosphate is an intermediate and is shown below. 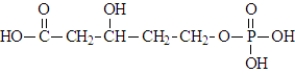 At physiological pH this lipid intermediate would exist as:
At physiological pH this lipid intermediate would exist as:
(Multiple Choice)
4.8/5  (39)
(39)
One of the necessary components for fatty acid synthesis is a complex of malonic acid (shown below) and CoA. 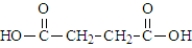 The structure of this complex at physiological pH would be:
The structure of this complex at physiological pH would be: 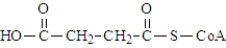
(True/False)
4.9/5  (35)
(35)
The water solubility of heptanoic acid is less than that of potassium heptanoate.
(True/False)
4.8/5  (38)
(38)
Consider the following nitrogen containing compounds. 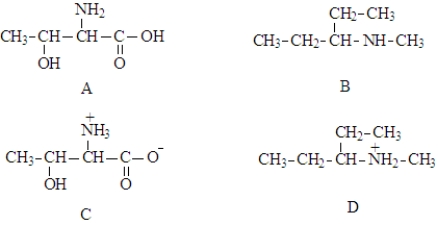 Fill in the blanks with the appropriate letter (A, B, C, D).
-Structure________________ would be the form present in a pH 2 solution.
Fill in the blanks with the appropriate letter (A, B, C, D).
-Structure________________ would be the form present in a pH 2 solution.
(Short Answer)
4.9/5  (34)
(34)
Certain amines called tryptamines, have an effect on the central nervous system and share a common structural feature.
(True/False)
5.0/5  (33)
(33)
Consider the structures given below.  Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure ___________________ would exist as a negative ion at pH 9.
Fill in the blanks with appropriate letter (A, B, C, D, E, F).
-Structure ___________________ would exist as a negative ion at pH 9.
(Short Answer)
4.9/5  (30)
(30)
When dimethylamine reacts with butanoic acid, which of the following is a product of the reaction?
(Multiple Choice)
4.8/5  (37)
(37)
The following illustrates an amine acting as hydrogen bond donor with water. 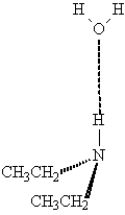
(True/False)
4.9/5  (29)
(29)
The following compounds are constitutional isomers. Which can function as only a hydrogen bond acceptor?
(Multiple Choice)
4.9/5  (35)
(35)
Imitrexwas developed in the late 1980's and is used in the treatment of migraine. The active ingredient is shown below. 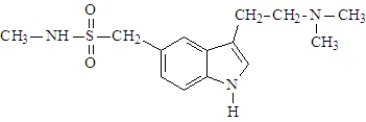 The substance would be classified as a(n):
The substance would be classified as a(n):
(Multiple Choice)
4.7/5  (26)
(26)
Showing 1 - 20 of 64
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)