Exam 7: Acids and Bases
Exam 1: Measurements in Science and Medicine77 Questions
Exam 2: Atoms, Elements, and Compounds77 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Energy and Physical Properties74 Questions
Exam 5: Solution Concentration77 Questions
Exam 6: Chemical Reactions68 Questions
Exam 7: Acids and Bases85 Questions
Exam 8: Nuclear Chemistry67 Questions
Exam 9: Hydrocarbons: an Introduction to Organic Molecules74 Questions
Exam 10: Hydration, Dehydration, and Alcohols61 Questions
Exam 11: Carbonyl Compounds and Redox Reactions71 Questions
Exam 12: Organic Acids and Bases64 Questions
Exam 13: Condensation and Hydrolysis Reactions72 Questions
Exam 14: Proteins67 Questions
Exam 15: Carbohydrates75 Questions
Exam 16: Lipids and Membranes79 Questions
Exam 17: Nucleic Acids, Protein Synthesis, and Heredity71 Questions
Select questions type
All of the following solutions are 0.025 M, which of the solutions contains the highest concentration of OH-?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
B
Which of the following is characteristic of a buffer?
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
B
Which of the following solutions is the most alkaline?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
D
NaOH and H2SO4 are mixed and allowed to react. How many grams of NaOH are required to neutralize 17.25 g of H2SO4?
(Short Answer)
4.9/5  (35)
(35)
If you dissolve 0.10 mol of a generic acid HA in 1.0 L of solution, the pH is 3.5. Is HA a strong or weak acid? Explain your answer.
(Essay)
4.8/5  (36)
(36)
A sodium hydroxide solution has a pH of 12.40, what is the OH- concentration in this solution?
(Multiple Choice)
4.7/5  (40)
(40)
When fumaric acid, H2C4H2O4 reacts with NaOH, the second reaction that occurs is
(Multiple Choice)
4.9/5  (36)
(36)
When a solution of HF (a weak acid) is added to a solution containing HPO42- (here, a base), the equation for reaction that occurs is:
(Multiple Choice)
4.9/5  (30)
(30)
Consider the following reaction. CH3OH(l) + CH3OH(l) 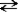 CH3O-(l) + CH3OH2+(l)
This reaction could be classified as both an autoionization reaction and a proton transfer reaction.
CH3O-(l) + CH3OH2+(l)
This reaction could be classified as both an autoionization reaction and a proton transfer reaction.
(True/False)
4.7/5  (37)
(37)
When a solution of KOH is added to a solution of HCO2H (formic acid), which of the following would be shown in the molecular equation as a product of the reaction?
(Multiple Choice)
4.7/5  (36)
(36)
Write the equation for the ionization of the weak acid, formic acid, HCHO2, in water.
(Essay)
4.8/5  (42)
(42)
When LiOH reacts with HNO3, the molecular equation for the reaction is:
(Multiple Choice)
4.9/5  (35)
(35)
In an aqueous solution. the [OH-] is 2.0 × 10-3 M. What is the [H3O+] of this solution?
(Multiple Choice)
4.7/5  (42)
(42)
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants: Products: 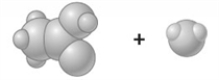
 If the composition of the equilibrium mixture is as represented below
If the composition of the equilibrium mixture is as represented below 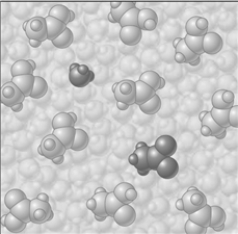 CH3COOH is classified as
CH3COOH is classified as
(Multiple Choice)
4.9/5  (35)
(35)
The following solutions are ranked from the most acidic to the most basic. Most acidic urine (pH 5.89) intestine contents (pH 8.06) blood (pH 7.30) Most basic
(True/False)
4.7/5  (47)
(47)
Which of the following is true of a buffer prepared with equal concentrations of an acid and its conjugate base?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)