Exam 5: Chemistry of Bonding: Structure and Function of Drug Molecules
Exam 1: Understanding Our World With Chemistry129 Questions
Exam 2: Matter: Properties, Changes and Measurements126 Questions
Exam 3: Understanding Atoms120 Questions
Exam 4: Bonding and Reactions112 Questions
Exam 5: Chemistry of Bonding: Structure and Function of Drug Molecules123 Questions
Exam 6: Aqueous Solutions: Part I118 Questions
Exam 7: Aqueous Solutions: Part II122 Questions
Exam 8: Organic Chemistry and Polymers123 Questions
Exam 9: Chemistry of Fire and Heat122 Questions
Exam 10: Chemistry of Explosions124 Questions
Exam 11: Applications of Chemical Kinetics124 Questions
Exam 12: Nuclear Chemistry: Energy, Medicine, Weapons, and Terrorism122 Questions
Exam 13: Chemical Equilibrium125 Questions
Exam 14: Introduction to Biochemistry118 Questions
Select questions type
What is the term for two molecules with the same formula but two different structures?
(Short Answer)
4.8/5  (31)
(31)
How does Lewis theory explain the loss or gain of electrons in an ionic bond?
(Multiple Choice)
4.8/5  (34)
(34)
What do the Lewis structures of all the alkali metals have in common?
(Multiple Choice)
4.9/5  (36)
(36)
The following molecule, phenol, is used in some medicines. How many carbon atoms in it have a tetrahedral geometry?
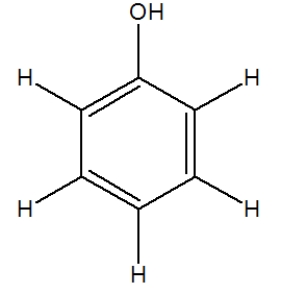
(Multiple Choice)
4.9/5  (29)
(29)
What is the proper term for the nerve cells that make up the human nervous system?
(Short Answer)
4.8/5  (35)
(35)
Lewis structures with single lines indicate what kind of bonding?
(Multiple Choice)
4.8/5  (48)
(48)
How many carbons in the following molecule have a tetrahedral geometry?
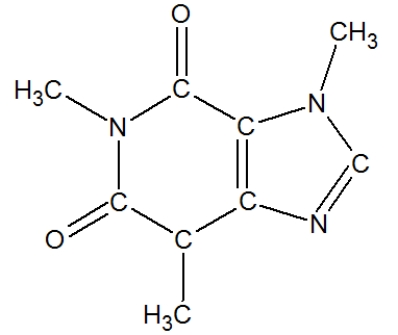
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following represent the best structure for SO2?
(Multiple Choice)
4.9/5  (29)
(29)
What kind of bond connects the carbon atom and oxygen atom in carbon monoxide?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following would be correct structure for the nitrite ion, NO2- ?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 101 - 120 of 123
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)