Exam 12: Oxidation-Reduction Reactions
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Which of the following pairs of ions cannot coexist in solution because a spontaneous redox reaction occurs?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
B
 Determining Oxidation Numbers
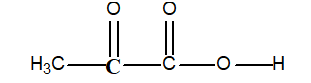
-Determine the oxidation number of the bold face carbon atom in pyruvic acid:
Determining Oxidation Numbers
-Determine the oxidation number of the bold face carbon atom in pyruvic acid:
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
B
 Determining Oxidation Numbers
-Determine the oxidation state of the carbon atom in bold print:
CH3CH2F
Determining Oxidation Numbers
-Determine the oxidation state of the carbon atom in bold print:
CH3CH2F
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
E
Cobalt(III) oxide reacts with hydrogen gas to form cobalt metal and water.
Co2O3(s) + 3 H2(g) 2 Co(s) + 3 H2O(g)
What does this tell you about the relative strength of the oxidizing and reducing agents in this reaction?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following isn't an example of an oxidation-reduction reaction?
(Multiple Choice)
4.9/5  (30)
(30)
An electric current is passed through a solution of CuSO4(aq) producing Cu(s) at the cathode and O2(g) at the anode. If 3.48 L of O2(g) measured at STP is produced at the anode, how many grams of Cu(s) must have been deposited on the cathode?
(Multiple Choice)
4.9/5  (45)
(45)
Calculate the complex dissociation equilibrium constant (Kd) for the Cd(NH3)42+ complex from the following data at 298K. 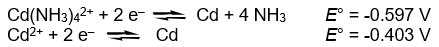
(Multiple Choice)
4.7/5  (38)
(38)
A molten sample of TiCl4 was electrolyzed for 10.0 hours at 12 amps. What is the ratio of the weight of Cl2 produced compared to that of Ti?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is the correct half-cell reaction for the anode process in the electrolysis of an aqueous solution of potassium sulfate?
(Multiple Choice)
4.7/5  (38)
(38)
Use a table of standard reduction potentials to determine which of the following statements is true for the electrochemical cell diagrammed below. 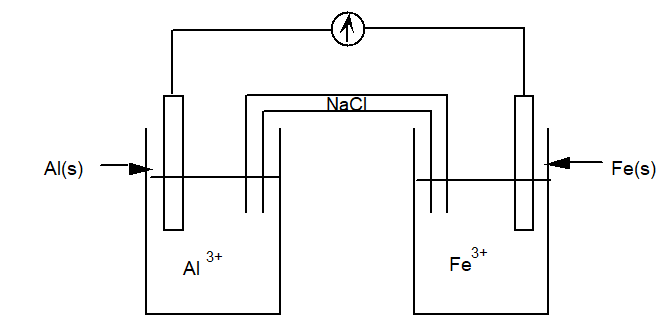
(Multiple Choice)
4.9/5  (35)
(35)
Use the following half reactions and accompanying standard reduction potentials to determine the best reducing agent. 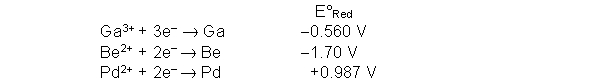
(Multiple Choice)
4.7/5  (41)
(41)
What is the ratio by weight of Br2 to Cr if a molten sample of CrBr2 is electrolyzed for 4.00 hr. at 10.0 amps?
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the weight of sodium metal that would be produced by the electrolysis of molten sodium chloride for 1.00 hour with a 10.0-amp current.
(Short Answer)
4.8/5  (39)
(39)
Calculate the amount of aluminum produced in 1.00 hour by the electrolysis of molten AlCl3 if the current is 10.0 A.
(Short Answer)
4.9/5  (35)
(35)
Which of the following electrolysis processes will produce the largest volume of Cl2 gas at STP?
(Multiple Choice)
4.8/5  (34)
(34)
What is the oxidation state of the osmium atom in an unknown salt if 26.7 grams of osmium plate out when a current of 15.0 amps is passed through a solution of this salt for 1.00 hour?
(Multiple Choice)
4.8/5  (37)
(37)
What is the magnitude of the standard-state cell potential for the following redox reaction?
2 Al(s) + 3 Pb2+(aq) 2 Al3+(aq) + 3 Pb(s)
(Multiple Choice)
5.0/5  (44)
(44)
Use a table of standard reduction potentials to determine which is the strongest oxidizing agent among the following.
(Multiple Choice)
4.9/5  (34)
(34)
refer to the following reaction which occurs in basic solution.
CrO42- + PH3 Cr(OH)4- + P4
-How many CrO42- ions are consumed in the balanced chemical equation?
(Multiple Choice)
4.8/5  (34)
(34)
Write a balanced chemical equation for the following reaction, which can be used to standardize aqueous permanganate ion solutions.
H2C2O4(aq) + MnO4-(aq) + H+(aq) CO2(g) + Mn2+(aq)
(Essay)
4.8/5  (41)
(41)
Showing 1 - 20 of 81
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)