Exam 4: Extraction
Consider the following solvent pairs. If mixed together, which pairs would form two layers? If they form two layers, which solvent would be on top?
(a) hexanes and water
(b) water and methylene chloride
(c) hexanes and methylene chloride
(d) methanol and hexanes
(e) ethanol and water
(f) acetone and toluene
(a) Would form two layers; the hexanes (d=0.7) would be on top (water, d=1.0).
(b) Would form two layers; the water (d=1.0) would be on top (methylene chloride; d=1.3)
(c) Would not form two layers (miscible).
(d) Would form two layers; the hexanes (d=0.7) would be on top (methanol, d=0.79)
(e) Would not form two layers (miscible).
(f) Would not form two layers (miscible).
The pain reliever phenacetin is soluble in cold water to the extent of 1.0 g / 1310 mL and soluble in diethyl ether to the extent of 1.0 g / 90 mL.
(a) Determine the approximate distribution coefficient for phenacetin in these two solvents.
(b) If 50 mg of phenacetin were dissolved in 100 mL of water, how much ether would be required to extract 90% of the phenacetin in a single extraction?
(c) What percent of the phenacetin would be extracted from the aqueous solution in (b) by two 25-mL portions of ether?
(a) K (ether / water) = (1.0 g / 90 mL) / (1.0 g / 1310 mL) = 14.6
(b) K = (0.045 g / X mL ether) / (0.05 g / 100 mL water) = 14.6; X = 6.2 mL
(c) first extraction: (X / 25 mL) / ((50-X) / 100 mL = 14.6
X = 39 mg in ether
second extraction: 9 mg in ether
Total: (39 + 9) mg = 48 mg (96%)
You have prepared an organic compound by reacting it with strong aqueous acid. The workup procedure calls for extraction of the compound into methylene chloride, then an extraction of the methylene chloride solution with 5% NaHCO3.
(a) What is the purpose of the extraction with 5% NaHCO3?
(b) What precautions should you take when you add the 5% NaHCO3 to the organic solution?
(a) The sodium bicarbonate serves to neutralize any residual acid.
(b) Carbon dioxide gas (CO2) will evolve when the solutions are mixed and build up pressure in the separatory funnel. Be sure to vent frequently with the separatory funnel pointing away from people.
What is wrong with the following procedure?
"The reaction mixture, consisting of NaBr and an ethanol solution of the product, is diluted with an equal volume of water, then extracted once with an equal volume of diethyl ether. The lower aqueous layer is discarded."
Suppose you added an additional 50 ml of water to a separatory funnel containing a com- pound distributed between 50 mL of ether and 50 mL of water. (The compound you want is more soluble in ether than it is in water.) How will this addition affect
(a) the distribution coefficient of the compound?
(b) the actual distribution of the compound?
Complete the following equations. If no appreciable reaction occurs, write "no reaction."
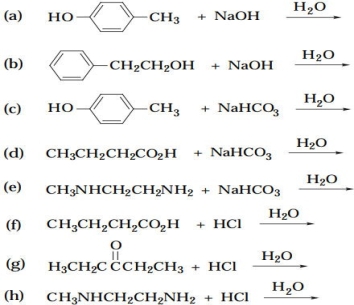
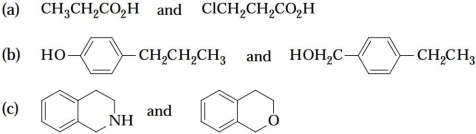
Which of the following pairs of compounds could be separated by chemically active extraction? What reagent would you use?
An aqueous solution containing 5.0 g of solute in 100 mL is extracted with three 25-mL por- tions of diethyl ether. What is the total amount of solute that will be extracted by the ether in each of the following cases?
(a) Distribution coefficient (ether-water), K = 0.10
(b) K = 1.0
(c) K = 10
You carefully purified phenanthrene from a very long, complicated procedure. It took days. It is very important for you to hand the purified phenanthrene in to your instructor in one hour to get a good grade. Oops! You accidentally put the phenanthrene into a vial that you thought was clean but instead had a lot of NaCl clinging to the sides. What can you do to quickly purify the phenanthrene again?
If the compound in problem 4 (b) were extracted with three 50-mL portions of diethyl ether, how much would be extracted?
Draw a flow diagram for the separation of CH3CH2CH2Br, (CH3CH2CH2)3N, and CH3CH2CO2H.
A reaction workup for an aqueous reaction mixture calls for extraction with diethyl ether and then an extraction with saturated aqueous sodium chloride. What is the purpose of the satu- rated sodium chloride step?
Diagram a two-stage dichloromethane (d = 1.3) extraction of an aqueous solution, showing how this procedure differs from the diethyl ether extraction diagrammed in Figure 3.8.
The distribution coefficients for a compound are:
benzene-water, K = 5.5
petroleum ether-water, K = 5.0
methylene chloride-water, K = 1.0
Which is the best choice of solvent for extracting the compound from an aqueous reaction mix- ture? Explain your
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)