Exam 10: Sublimation
Exam 1: Introduction to the Organic Laboratory16 Questions
Exam 2: Crystallization11 Questions
Exam 3: Melting Points10 Questions
Exam 4: Extraction14 Questions
Exam 5: Drying Organic Solutions6 Questions
Exam 6: Simple Distillation10 Questions
Exam 7: Fractional Distillation8 Questions
Exam 8: Vacuum Distillation6 Questions
Exam 9: Steam Distillation6 Questions
Exam 10: Sublimation4 Questions
Exam 11: Refractive Index3 Questions
Exam 12: Column Chromatography7 Questions
Exam 13: Thin Layer Chromatography10 Questions
Exam 14: Gas Chromatography10 Questions
Exam 15: Carrying Out Typical Reactions8 Questions
Exam 16: Infrared Spectroscopy6 Questions
Exam 17: Proton Nuclear Magnetic Resonance Spectroscopy8 Questions
Exam 18: The Chemical Literature8 Questions
Select questions type
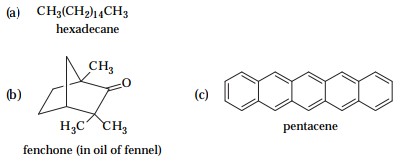
3 Which of the following compounds would be likely to evaporate readily? Explain your answer.
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
Only (b) and (c). Fenchone will evaporate readily because it is spherically shaped. Pentacene will evaporate becaue it is cylindricaly shaped. Hexadecane has a zig-zag shape and strong van der Waal attractions.
Which of the following compounds could be subjected to sublimation at atmospheric pres- sure?
a) Compound A: vapor pressure at its melting point = 770 mm Hg
(b) Compound B: vapor pressure at its melting point = 400 mm Hg
(c) Compound C: vapor pressure at its melting point = 10 mm Hg
Free
(Essay)
4.7/5  (41)
(41)
Correct Answer:
Compounds A and B will have significant vapor pressure at their melting point.
You have a mixture of p-nitrobenzaldehyde (vapor pressure 0.01 at its melting point of 106˚) and camphor. Explain how you would separate these two compounds by sublimation. How would you perform the sublimation if benzoic acid instead of camphor is used?
Free
(Essay)
4.8/5  (37)
(37)
Correct Answer:
Subject the mixture of p-nitrobenzaldehyde and camphor to atmospheric sublimation; the camphor will sublime onto the tube filled with ice while the p-nitrobenzaldehyde remains in the flask itself. If benzoic acid is used, the mixture could be separated similarly using vacuum sublimation.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)