Exam 1: Introduction to the Organic Laboratory
Exam 1: Introduction to the Organic Laboratory16 Questions
Exam 2: Crystallization11 Questions
Exam 3: Melting Points10 Questions
Exam 4: Extraction14 Questions
Exam 5: Drying Organic Solutions6 Questions
Exam 6: Simple Distillation10 Questions
Exam 7: Fractional Distillation8 Questions
Exam 8: Vacuum Distillation6 Questions
Exam 9: Steam Distillation6 Questions
Exam 10: Sublimation4 Questions
Exam 11: Refractive Index3 Questions
Exam 12: Column Chromatography7 Questions
Exam 13: Thin Layer Chromatography10 Questions
Exam 14: Gas Chromatography10 Questions
Exam 15: Carrying Out Typical Reactions8 Questions
Exam 16: Infrared Spectroscopy6 Questions
Exam 17: Proton Nuclear Magnetic Resonance Spectroscopy8 Questions
Exam 18: The Chemical Literature8 Questions
Select questions type
What volume of 50% NaOH is needed to prepare 25 mL of 2.0% NaOH?
Free
(Essay)
4.9/5  (36)
(36)
Correct Answer:
(a) 25 mL x (2.0 g / 100 mL) = 0.50 g
X mL x (50 g / 100 mL) = 0.50 g, X = 1 mL
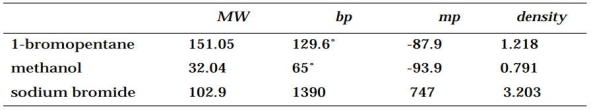
Look up the physical properties of the following compounds:
(a) 1-Bromopentane
(b) Methanol
(c) Sodium bromide
Free
(Essay)
4.9/5  (40)
(40)
Correct Answer:
Why is it important to clean up any chemical that you spill in the laboratory? If you do not know the identity of the chemical, where should you put it?
Free
(Essay)
4.9/5  (36)
(36)
Correct Answer:
Chemical spills create hazardous waste which must be properly disposed according the rules of the locality as set by university departments and state and local municipalities. If spills are not cleaned up, they might harm the next person to use the lab room. If you do not know the identity of a spilled chemical, ask the instructor where it should be placed for proper disposal.
Many student laboratories employ heating mantles for heating reaction mixtures. These devices should not be plugged directly into the electrical outlet. Why?
(Essay)
4.7/5  (33)
(33)
You have a summer job as a laboratory technician at a local chemical manufacturing company. What information about safety plans and the chemicals you will be working with should your company or supervisor provide?
(Essay)
4.9/5  (43)
(43)
What would be the heat source (or heat sources) of choice for boiling each of the following sol- vents?
(a) Diethyl ether, (CH3CH2)2O, bp 35˚
(b) Water, bp 100˚
(c) Ethanol, CH3CH2OH, bp 78˚
(d) Acetone, (CH3)2C=O, bp 56˚
(Essay)
4.9/5  (26)
(26)
What should you do in each of the following circumstances?
(a) Your neighbor splashes a chemical into his or her eye.
(b) A strong acid spills onto your hands.
(c) You spill a large amount of diethyl ether on the bench top.
(d) Your neighbor's clothing catches fire.
(e) Your reaction flask catches fire.
(Essay)
4.9/5  (40)
(40)
Find the following items in your student laboratory:
(a) Fire extinguishers.
(b) Safety wash or eye wash.
(c) EEP (Emergency Evacuation Plan).
(d) MSDS's.
(e) First aid kit.
(f) Closest phone for use in emergency situations.
(Essay)
4.9/5  (34)
(34)
Find the hazard information for each of the following compounds from three different sources, including one MSDS, one printed source, and one Web or online source. Compare and contrast the relative dangers of working with each chemical.
(a) Diethyl ether
(b) Benzene
(c) Methylene chloride
(d) Ethanol
(e) Benzophenone
(f) Sodium chloride
(Essay)
4.8/5  (48)
(48)
Give reasons for the following safety rules.
(a) Contact lenses should not be worn in the laboratory.
(b) A chemical spill on the skin should be washed off with water, not with solvent.
(c) Solvents are not to be poured down the sink.
(d) Water should not be used to extinguish laboratory fires.
(e) To dilute concentrated sulfuric acid, we pour it onto ice instead of simply mixing it with water.
(f) Broken glassware should be picked up immediately and put in the designated container.
(g) Closed-toe shoes should be worn to laboratory.
(Essay)
4.7/5  (35)
(35)
At the end of a reaction, your glassware is covered with a tarry substance. If you use acetone to clean the tar from the glassware, can you place the glassware immediately in a drying oven? Why or why not?
(Essay)
4.9/5  (32)
(32)
For each of the following reactions, (1) identify the limiting reagent, and (2) calculate the theo- retical yield of the organic product. (Note: The equations as shown are not necessarily bal- anced.)
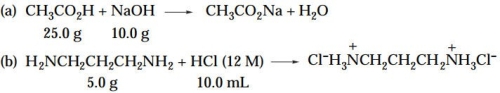
(Essay)
4.7/5  (34)
(34)
You open your laboratory drawer to prepare your glassware for an experiment. You find that your round-bottom flask is attached so firmly to your distillation adapter that you cannot take them apart.
(a) What should you have done when storing your glassware during the previous lab period?
(b) How can you get the two pieces of glassware apart?
(Essay)
4.9/5  (28)
(28)
Make the following conversions.5.0 g CH3CH2CH2CH2CH2Br to moles
(d) 0.100 mol CH3OH to grams
(e) 2.50 mol NaBr to grams
(f) 10.0 mL concd H2SO4 (96%, density 1.84) to moles.*
(g) 0.30 mol H2SO4 to mL of 6N H2SO4
(Essay)
4.8/5  (29)
(29)
You need 45 mL of a 5% aqueous solution of NaHCO3.
(a) What weight of NaHCO3 is required?
(b) How much water will you add?
(Essay)
4.8/5  (45)
(45)
Calculate the percent yield when:
(a) the theoretical yield of a product is 15.3 g and a student obtains 6.9 g
(b) the theoretical yield is 3.1 g and a student obtains 2.7 g
(Essay)
4.9/5  (33)
(33)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)