Exam 6: Some Processes Are Irreversible Thermal Physics
Exam 1: Conservation Laws Constrain Interactions216 Questions
Exam 2: The Laws of Physics Are Universal Newtonian Mechanics147 Questions
Exam 3: The Laws of Physics Are Frame-Independent Relativity124 Questions
Exam 4: Electricity and Magnetism Are Unified333 Questions
Exam 5: Matter Behaves Like Waves Quantum Physics210 Questions
Exam 6: Some Processes Are Irreversible Thermal Physics151 Questions
Select questions type
A heat engine produces of mechanical power while discarding into the environment (its cold reservoir). What is this engine's efficiency?
(Multiple Choice)
4.9/5  (34)
(34)
Consider each of the following processes. Is the process consistent (C) or inconsistent (D) with the limitations on equation T8.12 (that be fixed and that the process not involve any energy transfer that is neither heat nor work due to a quasistatic volume change)?
-c) A pan of water boils on the stove.
(Multiple Choice)
5.0/5  (39)
(39)
A mole of iron has about half the mass of a mole of silver. Which likely has the greater heat capacity?
(Multiple Choice)
4.9/5  (40)
(40)
Suppose that the forces holding an atom in its position in a crystal are the same for two different monatomic solids, but the mass of an atom is larger for solid than for solid . Which has the larger value of ?
(Multiple Choice)
4.7/5  (47)
(47)
Suppose that we have two containers, one holding molecules of helium gas, and one holding molecules of oxygen gas. Both are initially at room temperature. We then add the same amount of energy to each gas. Which is hotter at the end?
(Multiple Choice)
4.7/5  (44)
(44)
A refrigerator uses of electric power and discards of thermal power into the kitchen. What is its coefficient of performance?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following devices are heat engines? In each case, answer T (True) if it is a heat engine, and F (False) if it is not.
-(a) Your body
(True/False)
4.8/5  (44)
(44)
An atom of helium can store energy by bumping an electron from its lowest orbital energy state to a higher orbital energy level. In particular, moving an electron from the lowest state to the next-lowest state would store an energy of . Why can we ignore this energy storage mode when calculating the heat capacity of helium gas?
(Multiple Choice)
4.9/5  (32)
(32)
Doubling the number of effective opaque layers in a planet's atmosphere from to will make its average surface temperature increase by what factor?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the systems listed below is the largest, having and for which there is a better than 1 in a billion chance of seeing one or the other solid having zero energy? (Hint: Using StatMech to check cases is probably the easiest approach.)
(Multiple Choice)
4.9/5  (44)
(44)
How does the number of molecules with speed compare to the number having speed ?
(Multiple Choice)
4.8/5  (31)
(31)
As a normal object's thermal energy goes to zero, the slope of a graph of its entropy versus
(Multiple Choice)
4.8/5  (41)
(41)
Consider a rectangular book. The book is (approximately) symmetric for rotations around an axis that goes diagonally from one corner to the other
(Short Answer)
4.8/5  (31)
(31)
As the temperature difference between its reservoirs increases, the COP of a refrigerator
(Multiple Choice)
4.8/5  (36)
(36)
Consider a system whose multiplicity is 1 , no matter how much energy one puts into it. What is such a system's temperature?
(Multiple Choice)
4.9/5  (34)
(34)
Problems T7T. 3 through T7T. 7 refer to the following cyclic gas process:
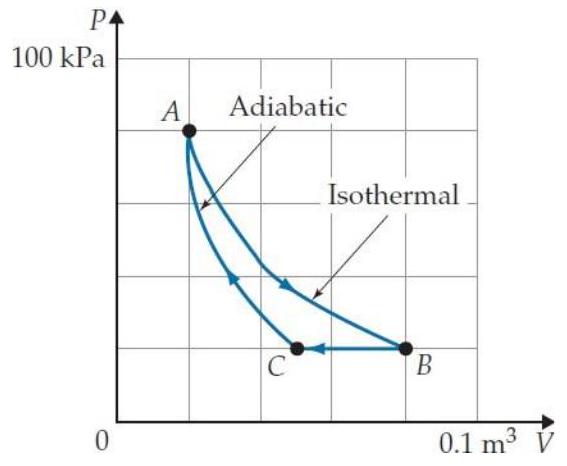 -In static equilibrium, any excess charge on a conductor is uniformly spread on its surface.
-In static equilibrium, any excess charge on a conductor is uniformly spread on its surface.
(Short Answer)
4.8/5  (43)
(43)
In each of the processes described below, energy flows across the boundary of the object that serves as the subject of each sentence. Is this energy flow heat (A), work (B), or some other kind of energy transfer (E)?
-(b) You get cooler when standing in the breeze from a fan.
(Short Answer)
5.0/5  (40)
(40)
Showing 81 - 100 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)