Exam 6: Some Processes Are Irreversible Thermal Physics
Exam 1: Conservation Laws Constrain Interactions216 Questions
Exam 2: The Laws of Physics Are Universal Newtonian Mechanics147 Questions
Exam 3: The Laws of Physics Are Frame-Independent Relativity124 Questions
Exam 4: Electricity and Magnetism Are Unified333 Questions
Exam 5: Matter Behaves Like Waves Quantum Physics210 Questions
Exam 6: Some Processes Are Irreversible Thermal Physics151 Questions
Select questions type
Classify the following hypothetical perpetual motion machines as being perpetual motion machines of the first kind or the second kind (B).
-(e) A normal heat engine is used to drive an electric generator. Part of the power from this generator runs a refrigerator that absorbs the waste heat from the engine and pumps it back into the hot reservoir.
(Multiple Choice)
4.9/5  (37)
(37)
Suppose that when we add to 1 mol of a certain gas, its temperature is observed to increase by . Which of the following statements is true?
(Multiple Choice)
4.9/5  (43)
(43)
Which system has the greater entropy, a puddle of water sitting on a table next to a chaotic scattering of salt crystals or the same amount of salt dissolved in an evenly distributed way in the puddle? Be prepared to describe your reasoning.
(Multiple Choice)
4.7/5  (41)
(41)
Suppose that we place objects and into a large bucket of water and allow them to come into equilibrium with the water. If we now extract and from the water and immediately place them in contact with each other, they will necessarily be in equilibrium.
(True/False)
4.9/5  (44)
(44)
Which of the following devices are heat engines? In each case, answer T (True) if it is a heat engine, and F (False) if it is not.
-(e) A rocket
(True/False)
4.9/5  (36)
(36)
Problems T7T. 3 through T7T. 7 refer to the following cyclic gas process:
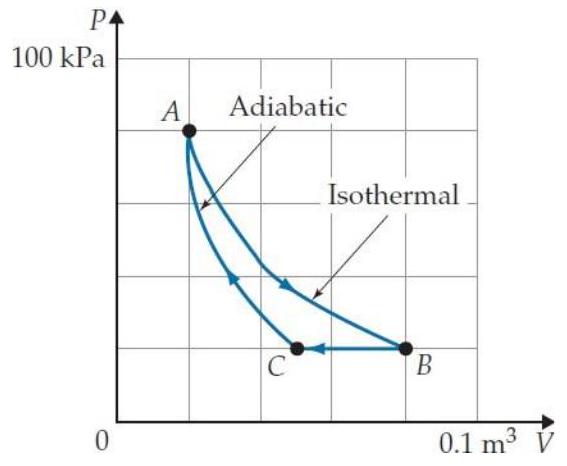 -The process shown is
-The process shown is
(Multiple Choice)
4.8/5  (30)
(30)
According to the text's final model (including the ocean effect), about when will the earth's average surface temperature be ?
(Multiple Choice)
4.9/5  (40)
(40)
Suppose we place a aluminum block with an initial temperature of in a Styrofoam cup containing a sample of water at . (The specific heats of aluminum and water are and , respectively.) The system's final temperature is closest to
(Multiple Choice)
4.9/5  (35)
(35)
Suppose that we double the electrical power flowing into the filament of an incandescent bulb. By what factor will the most probable photon energy increase?
(Multiple Choice)
4.8/5  (27)
(27)
A container holds 1 million helium molecules at . If we double the gas's temperature to , by about what factor does its multiplicity increase?
(Multiple Choice)
4.8/5  (30)
(30)
Suppose that an atom has exactly two energy levels separated by an energy difference . When such atoms are in contact with a reservoir at a temperature , the ratio of the number of atoms in the higher level to the number in the lower level is . If we increase the temperature to , the numerical value of this ratio
(Multiple Choice)
4.9/5  (39)
(39)
A hot object is placed in contact with a cold object. We observe that heat flows spontaneously from the hot object to the cold object, but not in the other direction. According to the argument in this chapter, this is so because
(Multiple Choice)
4.9/5  (39)
(39)
One can convert work to heat (the reverse of what a heat engine does) with perfect efficiency.
(True/False)
4.7/5  (40)
(40)
Suppose that we place an aluminum cylinder, a wooden block, and a Styrofoam cup on a table and leave them there for several hours. We then come back into the room and feel each object.
-(b) Which (if any) actually is coolest?
(Multiple Choice)
4.8/5  (48)
(48)
Problems T7T. 3 through T7T. 7 refer to the following cyclic gas process:
 -When an object slides down a frictionless incline, The interaction does not change the object's momentum
-When an object slides down a frictionless incline, The interaction does not change the object's momentum
(Short Answer)
5.0/5  (31)
(31)
Suppose that the temperature is about for a certain Einstein solid. The probability that an oscillator in that solid will have one unit of energy is closest to
(Hint: See equation for how to calculate .)
Equation T4.32:
(Multiple Choice)
4.9/5  (37)
(37)
All irreversible processes involve
-(b) transfers to an object's thermal energy,
(True/False)
4.9/5  (29)
(29)
In each of the processes described below, energy flows across the boundary of the object that serves as the subject of each sentence. Is this energy flow heat (A), work (B), or some other kind of energy transfer (E)?
-(e) Your pizza gets warm in the microwave.
(Short Answer)
4.9/5  (38)
(38)
Showing 121 - 140 of 151
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)